Principles of Flow Chemistry Overview What is flow


- Slides: 20

Principles of Flow Chemistry

Overview • What is flow chemistry? • Flow Chemistry vs Batch Chemistry • Key principles of Flow Chemistry • Residence Time • Mixing • Pressure • Temperature • Types of Flow Chemistry • Summary

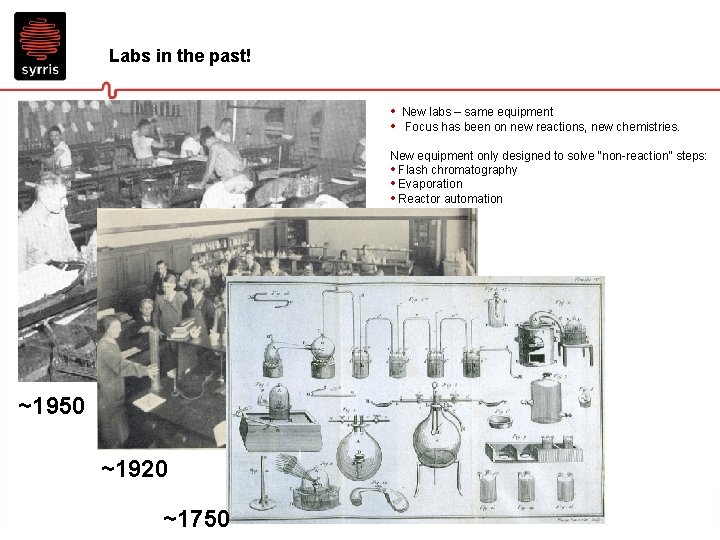
Labs in the past! • New labs – same equipment • Focus has been on new reactions, new chemistries. New equipment only designed to solve “non-reaction” steps: • Flash chromatography • Evaporation • Reactor automation ~1950 ~1920 ~1750

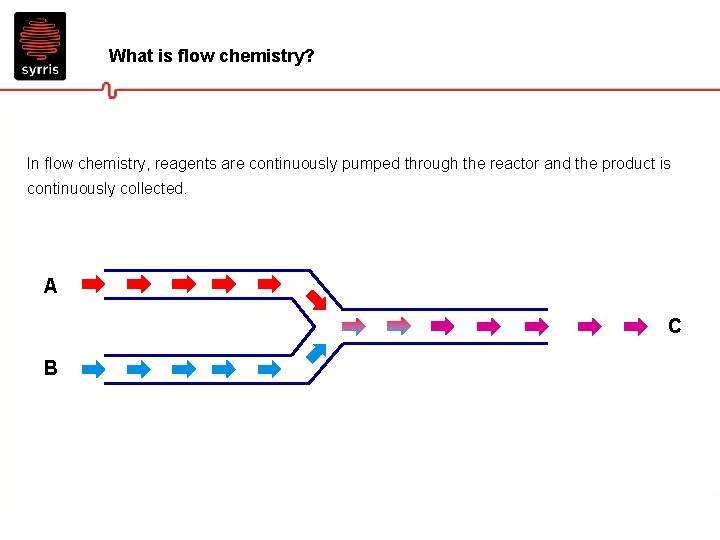
What is flow chemistry? In flow chemistry, reagents are continuously pumped through the reactor and the product is continuously collected. A C B

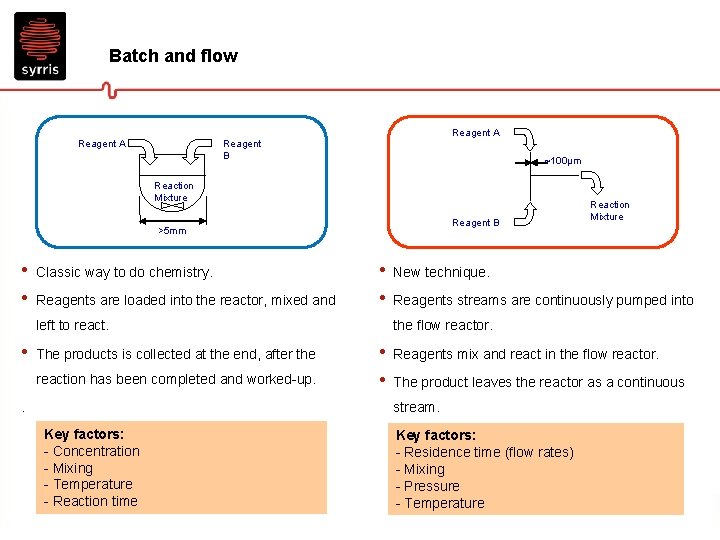
Batch and flow Reagent A Reagent B ~100µm Reaction Mixture Reagent B >5 mm • • Classic way to do chemistry. Reagents are loaded into the reactor, mixed and • • left to react. • The products is collected at the end, after the reaction has been completed and worked-up. New technique. Reagents streams are continuously pumped into the flow reactor. • • Reagents mix and react in the flow reactor. The product leaves the reactor as a continuous stream. . Key factors: - Concentration - Mixing - Temperature - Reaction time Reaction Mixture Key factors: - Residence time (flow rates) - Mixing - Pressure - Temperature

Key Principles of Flow Chemistry • Residence Time • Mixing • Pressure • Temperature

Residence time • It can be defined as the time that every fraction of the reaction volume spends in the reactor • Residence time is equivalent to reaction time in batch chemistry. • It is calculated as follows: Residence Time = Reactor Volume / Flow Rate Two ways of controlling the residence time: • Vary the reactor volume. • Vary the flow rates. Example: to achieve a longer residence time, it is possible to either pump more slowly and/or use a reactor with a larger volume.

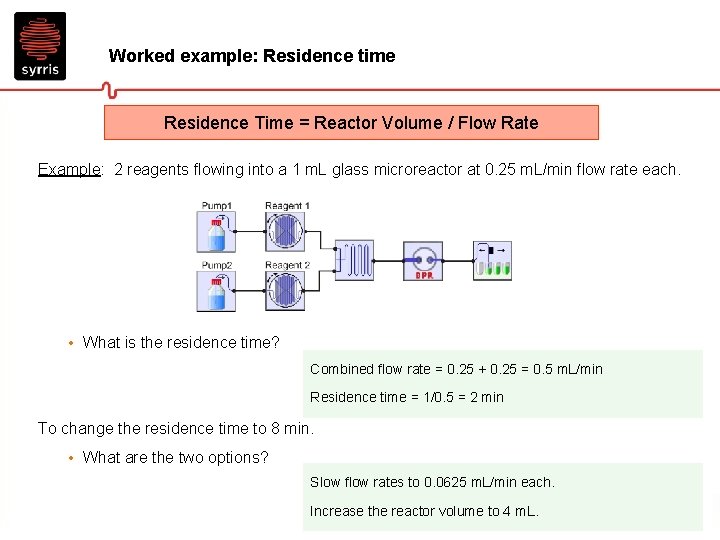
Worked example: Residence time Residence Time = Reactor Volume / Flow Rate Example: 2 reagents flowing into a 1 m. L glass microreactor at 0. 25 m. L/min flow rate each. • What is the residence time? Combined flow rate = 0. 25 + 0. 25 = 0. 5 m. L/min Residence time = 1/0. 5 = 2 min To change the residence time to 8 min. • What are the two options? Slow flow rates to 0. 0625 m. L/min each. Increase the reactor volume to 4 m. L.

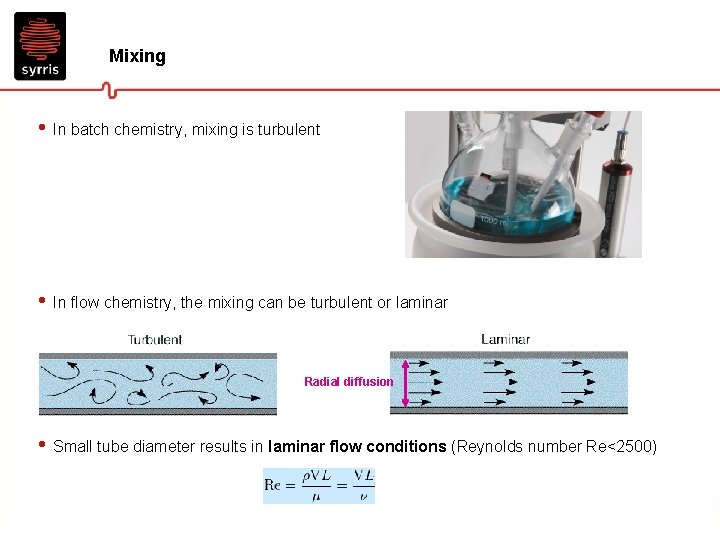
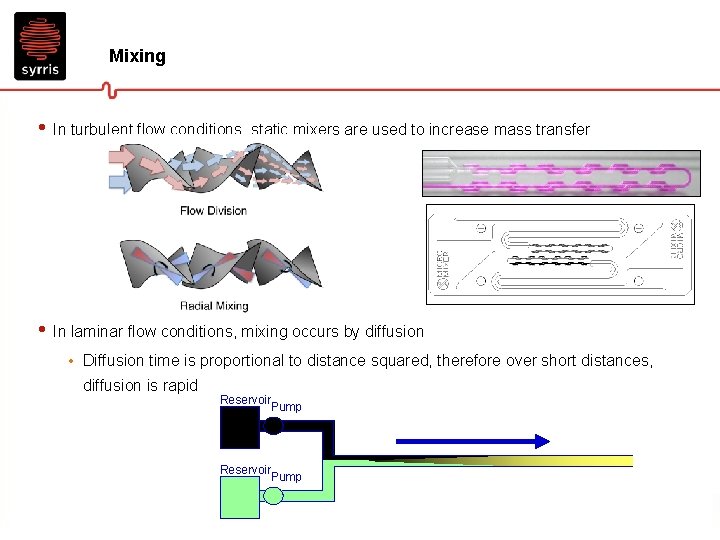
Mixing • In batch chemistry, mixing is turbulent • In flow chemistry, the mixing can be turbulent or laminar Radial diffusion • Small tube diameter results in laminar flow conditions (Reynolds number Re<2500)

Mixing • In turbulent flow conditions, static mixers are used to increase mass transfer • In laminar flow conditions, mixing occurs by diffusion • Diffusion time is proportional to distance squared, therefore over short distances, diffusion is rapid Reservoir Pump

Pressure • In a flow reactor the total pressure at any location is made up of two factors: • Back pressure due to flow • This increases with higher flow rate, narrower channels or more viscous liquid • Back pressure intentionally applied • This is typically applied by a pressure regulator near the exit of the system • Bubbles are best avoided as they can “push out” the reaction, thus lowering the residence time • Flow reactors can be easily pressurised (much easier than a batch reaction) • This can be useful for a variety of reasons: • Reactions with gas • Avoiding cavitation • Superheating

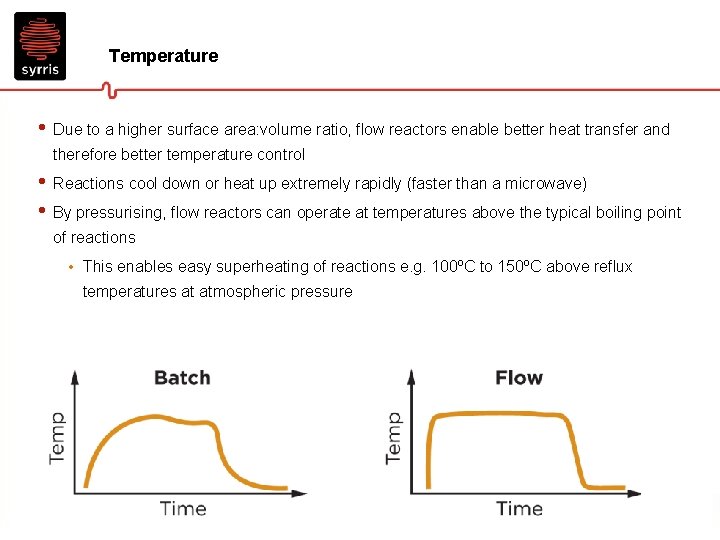
Temperature • Due to a higher surface area: volume ratio, flow reactors enable better heat transfer and therefore better temperature control • Reactions cool down or heat up extremely rapidly (faster than a microwave) • By pressurising, flow reactors can operate at temperatures above the typical boiling point of reactions • This enables easy superheating of reactions e. g. 100ºC to 150ºC above reflux temperatures at atmospheric pressure

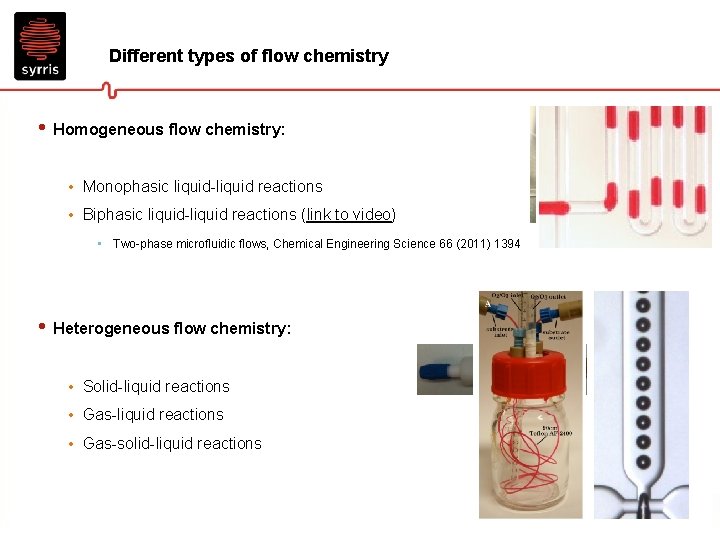
Different types of flow chemistry • Homogeneous flow chemistry: • Monophasic liquid-liquid reactions • Biphasic liquid-liquid reactions (link to video) • Two-phase microfluidic flows, Chemical Engineering Science 66 (2011) 1394 • Heterogeneous flow chemistry: • Solid-liquid reactions • Gas-solid-liquid reactions

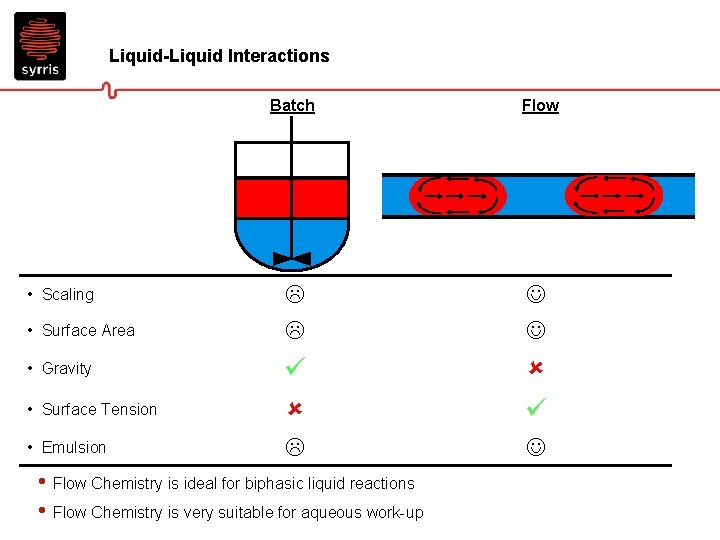
Liquid-Liquid Interactions Batch Flow • Surface Tension • Emulsion • Scaling • Surface Area • Gravity • Flow Chemistry is ideal for biphasic liquid reactions • Flow Chemistry is very suitable for aqueous work-up

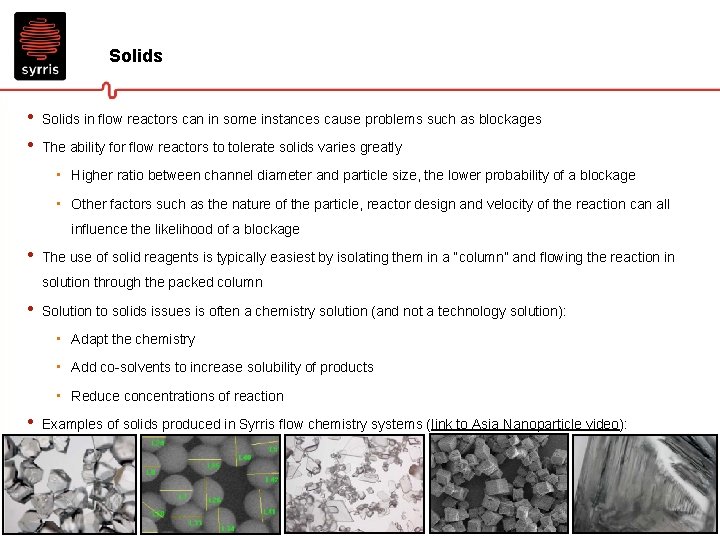
Solids • • Solids in flow reactors can in some instances cause problems such as blockages The ability for flow reactors to tolerate solids varies greatly • Higher ratio between channel diameter and particle size, the lower probability of a blockage • Other factors such as the nature of the particle, reactor design and velocity of the reaction can all influence the likelihood of a blockage • The use of solid reagents is typically easiest by isolating them in a “column” and flowing the reaction in solution through the packed column • Solution to solids issues is often a chemistry solution (and not a technology solution): • Adapt the chemistry • Add co-solvents to increase solubility of products • Reduce concentrations of reaction • Examples of solids produced in Syrris flow chemistry systems (link to Asia Nanoparticle video):

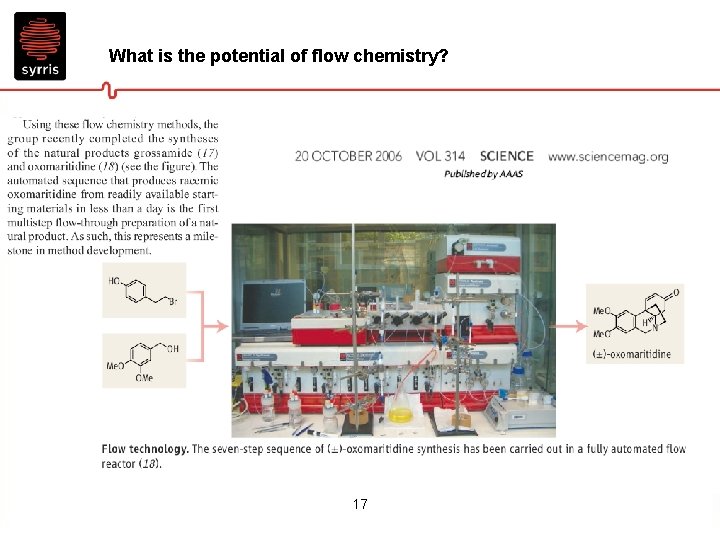
What is the potential of flow chemistry? 17

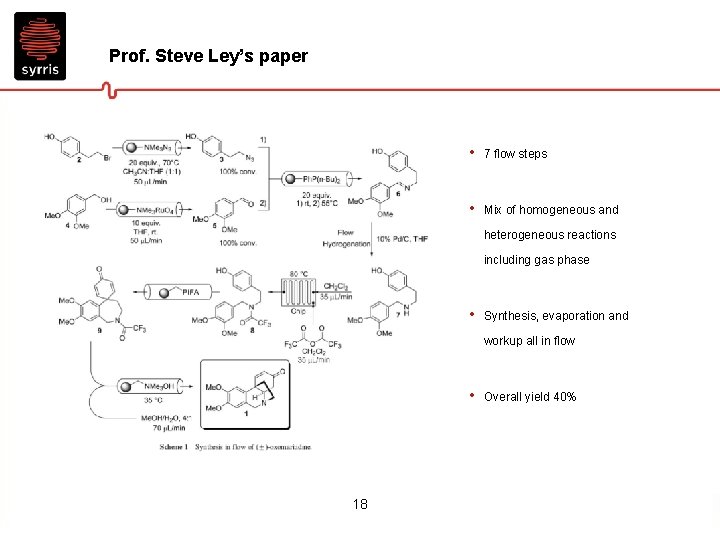
Prof. Steve Ley’s paper • 7 flow steps • Mix of homogeneous and heterogeneous reactions including gas phase • Synthesis, evaporation and workup all in flow • 18 Overall yield 40%

Examples of Syrris flow Chemistry Homogeneous catalysis Ring formations General Synthesis • Suzuki reaction • Grubbs ring forming • Aldol reaction • Heck reaction • Ugi followed by ring closure • Biphasic Schotten-Baumann • Grubbs ring forming to benzimidazole • HBTU amide coupling • Diels Alder • Elimination of an alcohol to alkene Multicomponent reactions • Passerini 3 CR • 1, 3, 4 Oxadiazole formation • Esterification of an alcohol • • Biginelli 3 CR • Fischer indole synthesis • Wittig reaction Ugi 4 CR • 1, 3 Thiazole formation • Nucleophilic aromatic substitution • Pyrazole formation • SN 1 reaction Deprotection chemistry • • • BOC deprotection Oxidations and reductions • Mitsunobu reaction MOM deprotection and intra • Borohydride reduction N-Alkylation epoxide opening • • Borane reduction of a Ester saponification heterocycle • Reductive amination • Dess Martin alcohol oxidation

Summary • Flow chemistry is an exciting new tool for chemists. • Reaction conditions: flow rates ratio, residence time, temperature. Residence Time = Reactor Volume / Combined Flow Rate • Variable parameters: flow rates, reactor volume, temperature • The technology is growing fast. • Later today you get a chance to see/use the most advanced flow chemistry systems available.

Any questions?