Principles of Chemotherapy and Chemotherapy Complications zlem Snmez




































- Slides: 73

Principles of Chemotherapy and Chemotherapy Complications Özlem Sönmez, MD Yeditepe University Hospital Section of Medical Oncology

Malignant cells

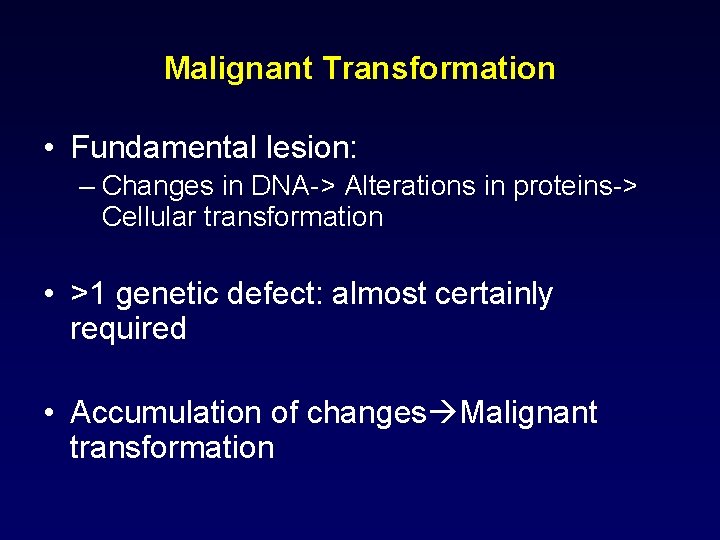
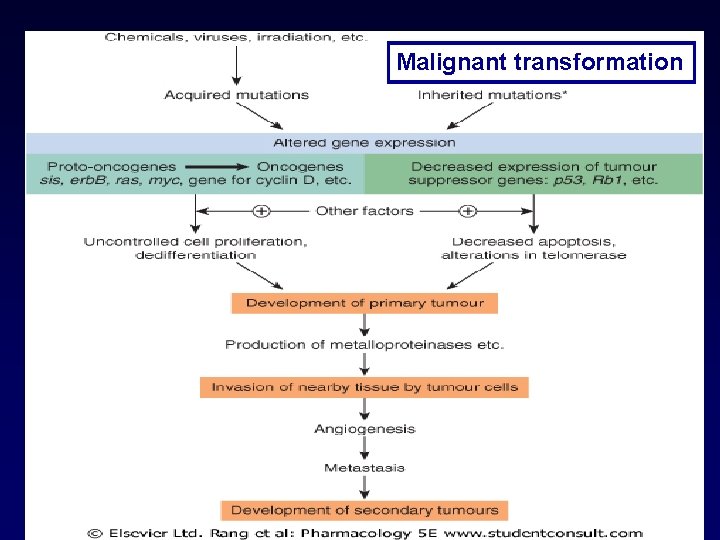
Malignant Transformation • Fundamental lesion: – Changes in DNA-> Alterations in proteins-> Cellular transformation • >1 genetic defect: almost certainly required • Accumulation of changes Malignant transformation

Malignant transformation 50. 2 Rang

Biological basis of cancer chemotherapeutics • Anticancer drugs interfere cellular processes that are altered in malignancy. • Antineoplastic effects – Cell death – Cell growth inhibited – Cell differentiation

• Cancer treatment usually involves one or more of surgery, radiotherapy and systemic therapy. • In early-stage disease, low-risk patients are often cured with surgery alone, but in many other cases a combination of treatments is required.

• In metastatic disease, systemic therapy is the principal therapeutic modality, as delivery through the blood stream facilitates access to disseminated cancer sites. • Systemic therapies include hormonal therapy, targeted therapy, and chemotherapy.

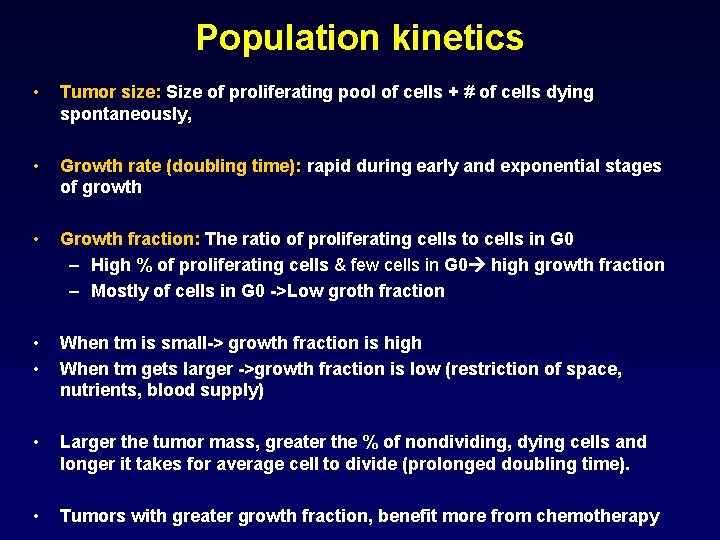
Population kinetics • Tumor size: Size of proliferating pool of cells + # of cells dying spontaneously, • Growth rate (doubling time): rapid during early and exponential stages of growth • Growth fraction: The ratio of proliferating cells to cells in G 0 – High % of proliferating cells & few cells in G 0 high growth fraction – Mostly of cells in G 0 ->Low groth fraction • • When tm is small-> growth fraction is high When tm gets larger ->growth fraction is low (restriction of space, nutrients, blood supply) • Larger the tumor mass, greater the % of nondividing, dying cells and longer it takes for average cell to divide (prolonged doubling time). • Tumors with greater growth fraction, benefit more from chemotherapy

Chemotherapy response • Solid tumors – Generally have a low growth fraction thus respond poorly to chemotherapy – In most cases need to be removed by surgery • Hematological tumors – Generally have a high growth fraction & generally respond well to chemotherapy

Population kinetics • 1 x 109 cells = 1 gr (1 cm) • 1 cell to 109 cells: 30 doubling times • Doubling time: 24 hours to years • 1012 -1013 cells: Damage to vital organs, death

Gompertzian Growth

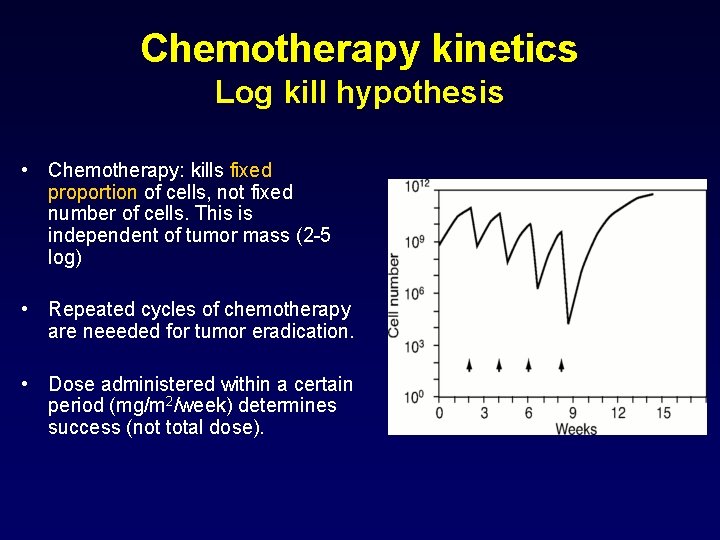
Chemotherapy kinetics Log kill hypothesis • Chemotherapy: kills fixed proportion of cells, not fixed number of cells. This is independent of tumor mass (2 -5 log) • Repeated cycles of chemotherapy are neeeded for tumor eradication. • Dose administered within a certain period (mg/m 2/week) determines success (not total dose).

Targets of Anticancer Drugs

Targets of Anticancer Drugs • All drugs have a target. • Traditional drug targets – DNA • • Nucleotide bases Enzymes of DNA synthesis Degradation Repair – Microtubules – GF receptors • Steroid hormone receptors

New targets • GFs (VEGF) • Mutated or overexpressed oncogene products – – – EGFR Her-2/neu (c-erb-B 2) Bcr: abl c. Kit b. Raf • Cell surface antigens – CD 33, CD 22, CD 20, CD 30, IL 2 -R • The machinery of protein synthesis (L-asparaginase) • Protein degradation (ubiquitin proteasomal degradation)

Targets • Drugs that affect these newer targets: “Targetted therapy” • Classic chemotherapeutics : Also have targets

Targets in Cancer Treatment

Classic chemotherapeutics

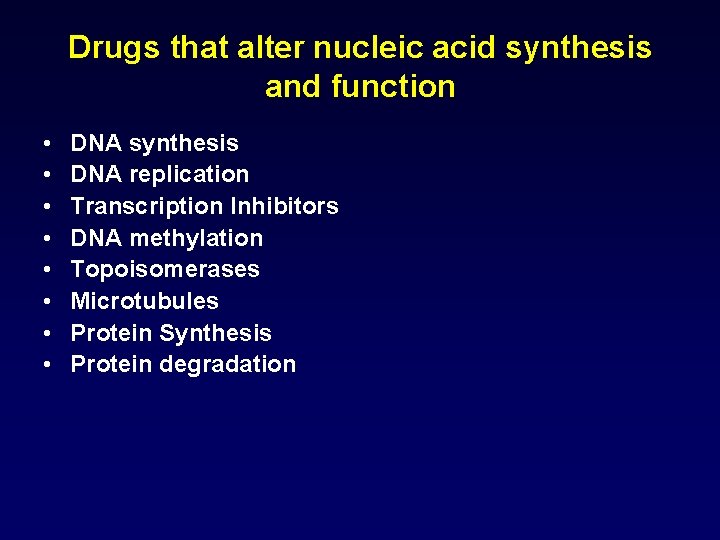
Drugs that alter nucleic acid synthesis and function • • DNA synthesis DNA replication Transcription Inhibitors DNA methylation Topoisomerases Microtubules Protein Synthesis Protein degradation

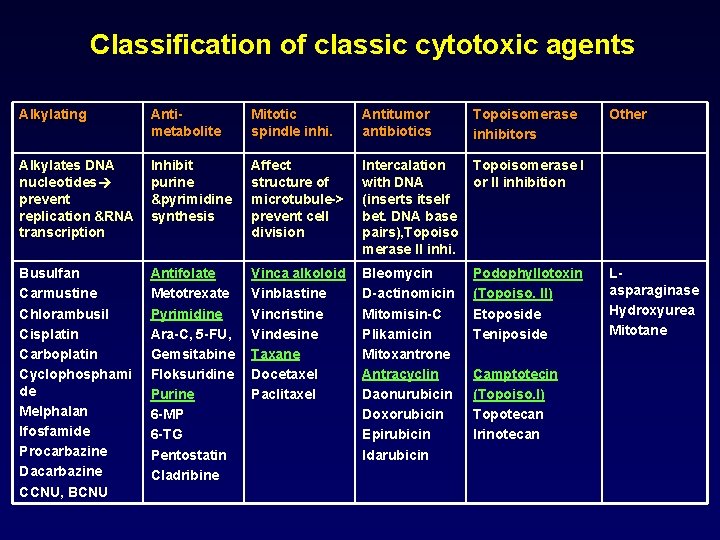
Classification of classic cytotoxic agents Alkylating Antimetabolite Mitotic spindle inhi. Antitumor antibiotics Topoisomerase inhibitors Alkylates DNA nucleotides prevent replication &RNA transcription Inhibit purine &pyrimidine synthesis Affect structure of microtubule-> prevent cell division Intercalation with DNA (inserts itself bet. DNA base pairs), Topoiso merase II inhi. Topoisomerase I or II inhibition Busulfan Carmustine Chlorambusil Cisplatin Carboplatin Cyclophosphami de Melphalan Ifosfamide Procarbazine Dacarbazine CCNU, BCNU Antifolate Metotrexate Pyrimidine Ara-C, 5 -FU, Gemsitabine Floksuridine Purine 6 -MP 6 -TG Pentostatin Cladribine Vinca alkoloid Vinblastine Vincristine Vindesine Taxane Docetaxel Paclitaxel Bleomycin D-actinomicin Mitomisin-C Plikamicin Mitoxantrone Antracyclin Daonurubicin Doxorubicin Epirubicin Idarubicin Podophyllotoxin (Topoiso. II) Etoposide Teniposide Camptotecin (Topoiso. I) Topotecan Irinotecan Other Lasparaginase Hydroxyurea Mitotane

Alkylating agents • Nitrogen mustards – Mechloroethamine – Cyclophosphamide – Ifosfamide – Chlorambucil – Melphalan • Alkylsulfonates – Busulfan • Nitrosoureas – Carmustine (BCNU) – Lomustine (CCNU) – Streptozocin – Semustine • Triazenes – Dacarbazine (DTIC) – Temozolamide • Hydrazines – Procarbazine • Platinum – Cisplatin – Carboplatin – Oxaliplatin

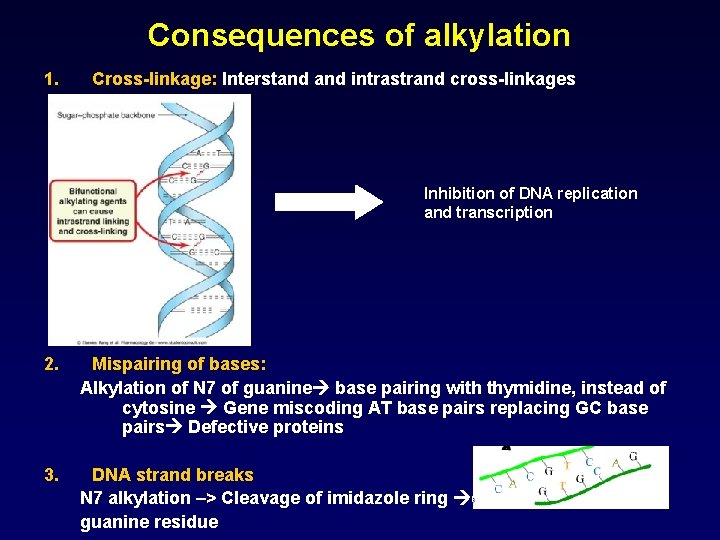
Alkylating agents Mechanism of action • Transfer alkyl groups to DNA • Alkylates nucleophilic groups on DNA bases, particularly at the N-7 position of guanine

Consequences of alkylation 1. Cross-linkage: Interstand intrastrand cross-linkages Inhibition of DNA replication and transcription 2. Mispairing of bases: Alkylation of N 7 of guanine base pairing with thymidine, instead of cytosine Gene miscoding AT base pairs replacing GC base pairs Defective proteins 3. DNA strand breaks N 7 alkylation –> Cleavage of imidazole ring excision of guanine residue

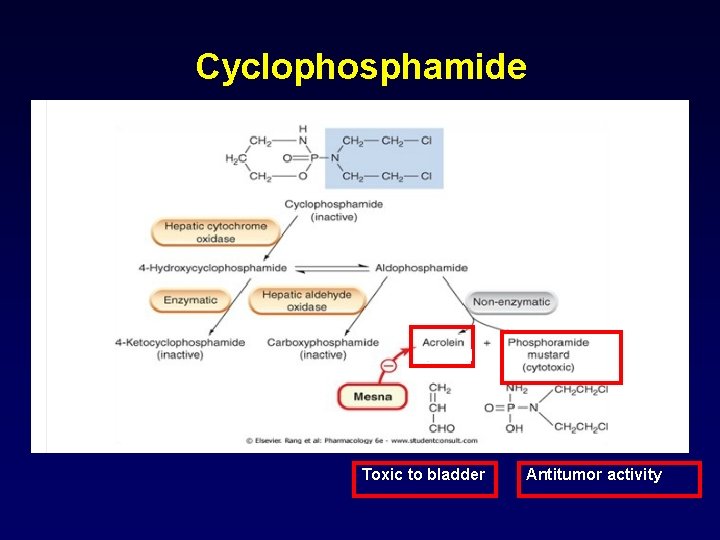
Cyclophosphamide Toxic to bladder Antitumor activity

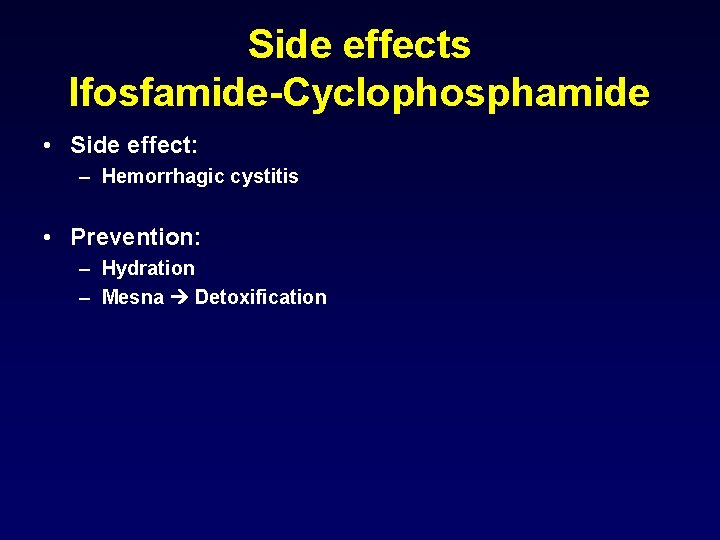
Ifosfamide • Analogue of cyclophosphamide • Metabolic activation to forn 4 -hydroxyifosfamide • More toxic to bladder Hemorrhagic cystitis

Side effects Ifosfamide-Cyclophosphamide • Side effect: – Hemorrhagic cystitis • Prevention: – Hydration – Mesna Detoxification

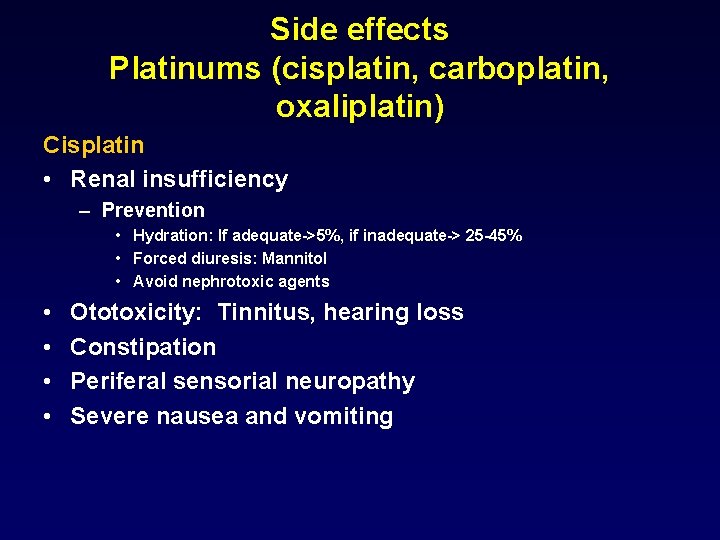
Side effects Platinums (cisplatin, carboplatin, oxaliplatin) Cisplatin • Renal insufficiency – Prevention • Hydration: If adequate->5%, if inadequate-> 25 -45% • Forced diuresis: Mannitol • Avoid nephrotoxic agents • • Ototoxicity: Tinnitus, hearing loss Constipation Periferal sensorial neuropathy Severe nausea and vomiting

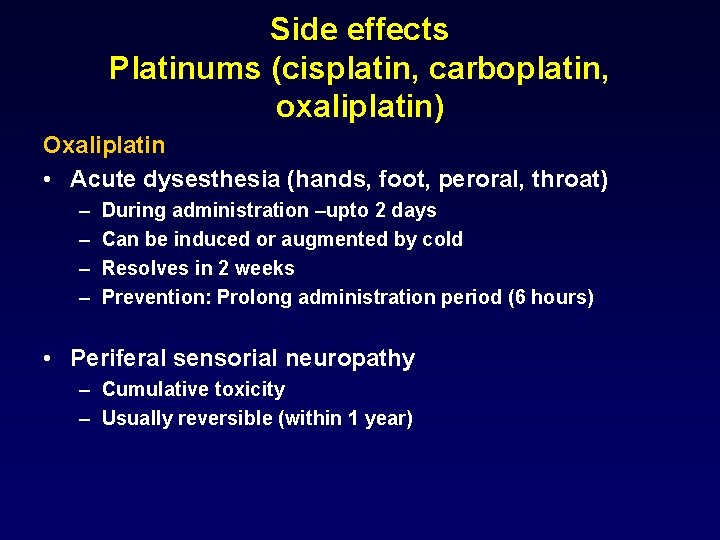
Side effects Platinums (cisplatin, carboplatin, oxaliplatin) Oxaliplatin • Acute dysesthesia (hands, foot, peroral, throat) – – During administration –upto 2 days Can be induced or augmented by cold Resolves in 2 weeks Prevention: Prolong administration period (6 hours) • Periferal sensorial neuropathy – Cumulative toxicity – Usually reversible (within 1 year)

Nitrosoureas • Carmustine (BCNU) • Lomustine (CCNU) • Highly lipid soluble Cross BBB

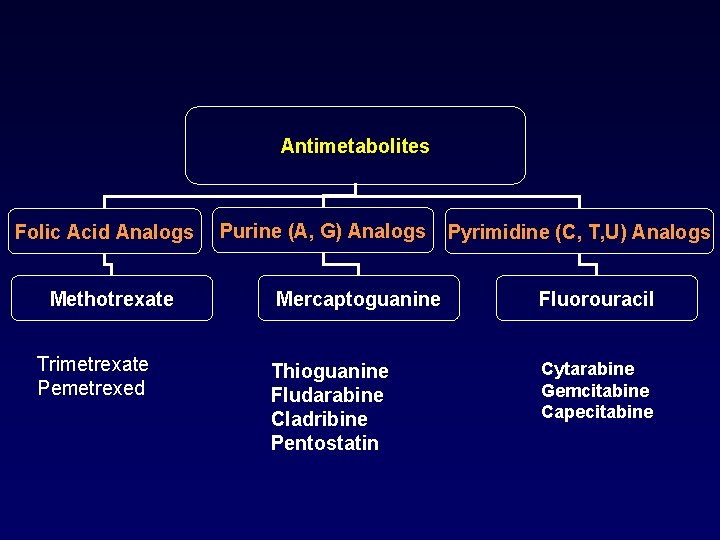
Antimetabolites Folic Acid Analogs Methotrexate Trimetrexate Pemetrexed Purine (A, G) Analogs Pyrimidine (C, T, U) Analogs Mercaptoguanine Fluorouracil Thioguanine Fludarabine Cladribine Pentostatin Cytarabine Gemcitabine Capecitabine

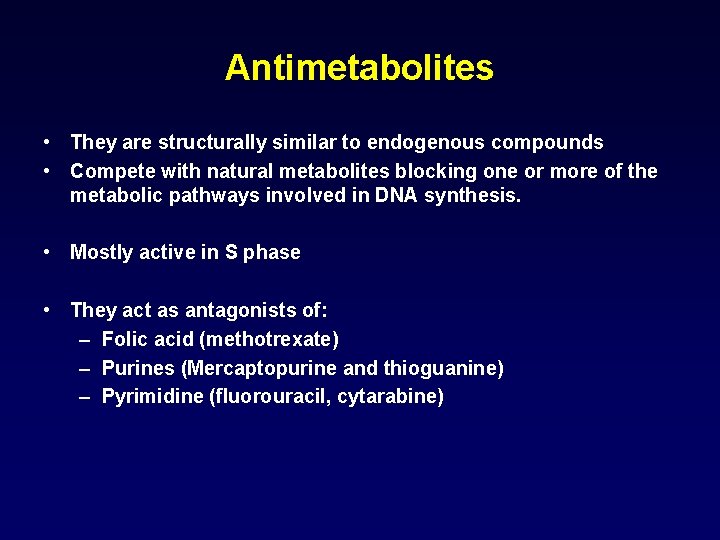
Antimetabolites • They are structurally similar to endogenous compounds • Compete with natural metabolites blocking one or more of the metabolic pathways involved in DNA synthesis. • Mostly active in S phase • They act as antagonists of: – Folic acid (methotrexate) – Purines (Mercaptopurine and thioguanine) – Pyrimidine (fluorouracil, cytarabine)

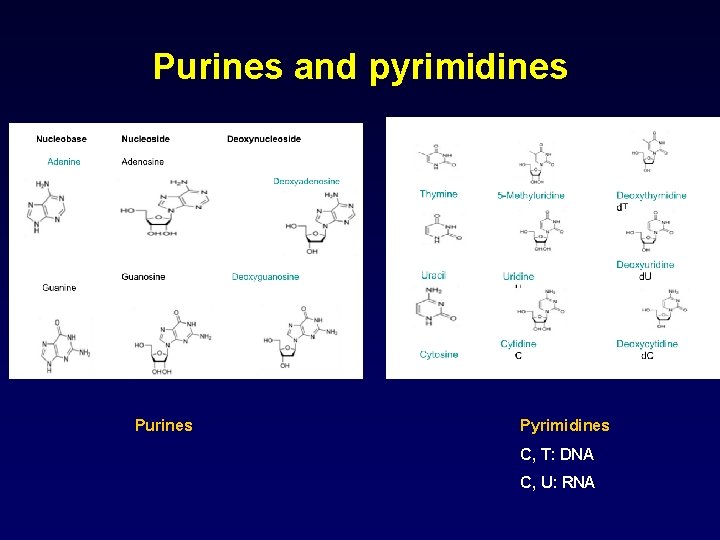
Purines and pyrimidines Purines Pyrimidines C, T: DNA C, U: RNA

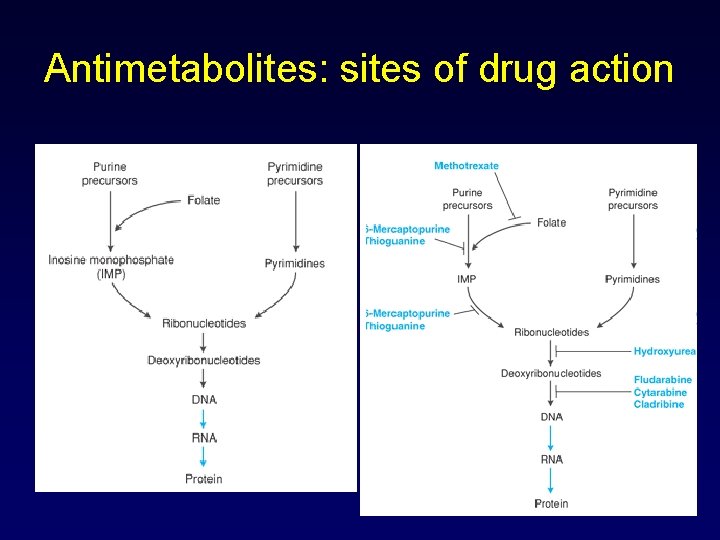
Antimetabolites: sites of drug action

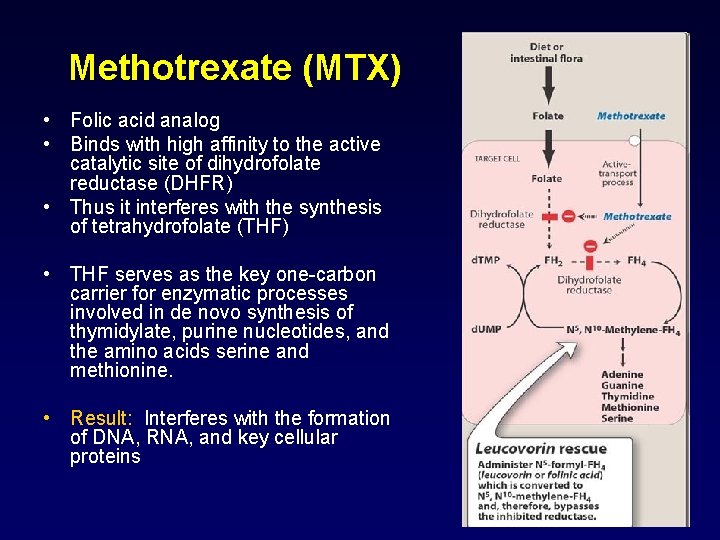
Methotrexate (MTX) • Folic acid analog • Binds with high affinity to the active catalytic site of dihydrofolate reductase (DHFR) • Thus it interferes with the synthesis of tetrahydrofolate (THF) • THF serves as the key one-carbon carrier for enzymatic processes involved in de novo synthesis of thymidylate, purine nucleotides, and the amino acids serine and methionine. • Result: Interferes with the formation of DNA, RNA, and key cellular proteins

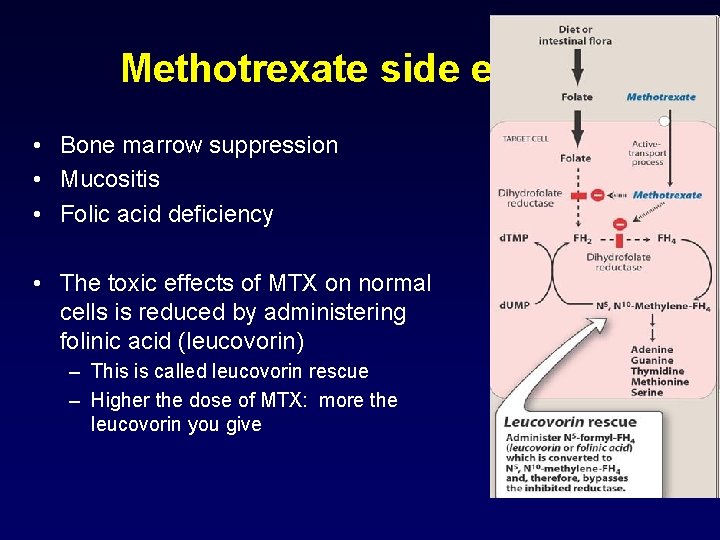
Methotrexate side effects • Bone marrow suppression • Mucositis • Folic acid deficiency • The toxic effects of MTX on normal cells is reduced by administering folinic acid (leucovorin) – This is called leucovorin rescue – Higher the dose of MTX: more the leucovorin you give

Side effects: Methotrexate • Side effects: – Mucositis – Folic acid deficiency • Prevention: – Hydration – Alkalinization of urine – Leukovorin rescue: 24 hours after treatment (calcium folinate)

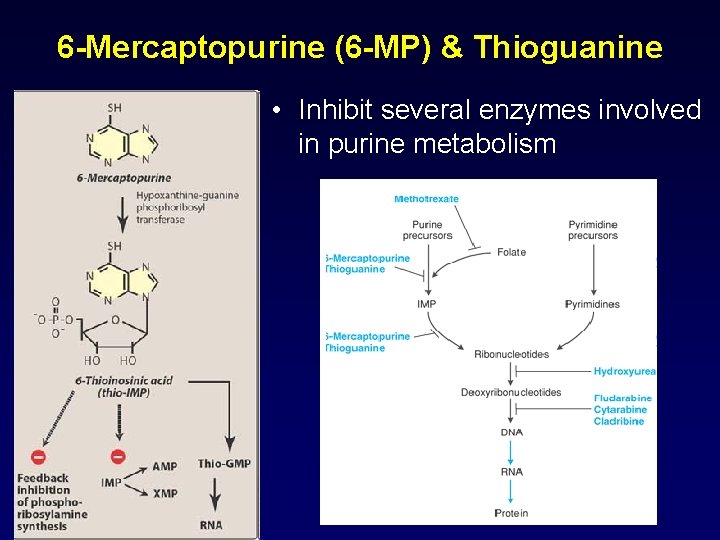
6 -Mercaptopurine (6 -MP) & Thioguanine • Inhibit several enzymes involved in purine metabolism

6 -MP & Allopurinol • 6 -MP: metabolized in the liver by xanthine oxidase and the inactive metabolites are excreted in the urine • Allopurinol (Ürikoliz) – Xanthine oxidase inhibitor – used to treat/prevent hyperuricemia • Do not use 6 -MP and allopurinol in combination. – If Allopurinol have to be used with 6 -MP, then the dose of 6 -MP is reduced by more than 75%

Cytarabine (Ara-C) • Cytarabine arabinoside is a pyrimidine antimetabolite • Inhibits conversion of cytidine to deoxycytidine • The drug is activated by kinases to Ara. CTP – This acts as an inhibitor of DNA polymerase • Side effect: At high doses cause neurotoxicity (cerebellar dysfunction and peripheral neuritis) – Hand-foot syndrome

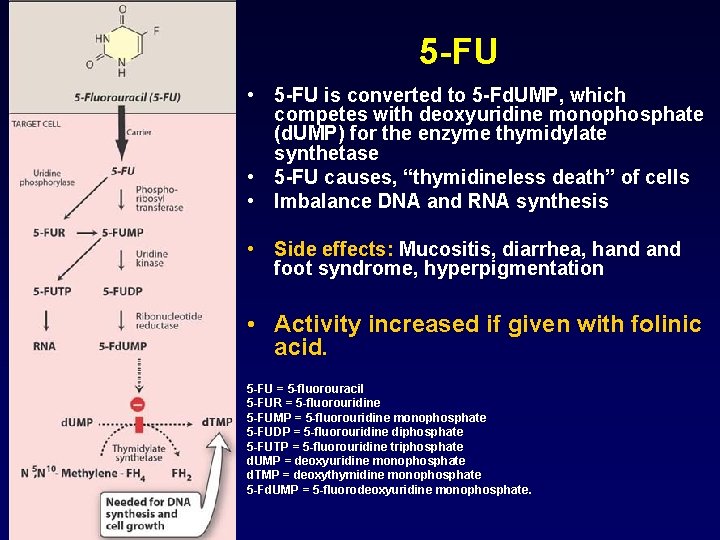
5 -FU • 5 -FU is converted to 5 -Fd. UMP, which competes with deoxyuridine monophosphate (d. UMP) for the enzyme thymidylate synthetase • 5 -FU causes, “thymidineless death” of cells • Imbalance DNA and RNA synthesis • Side effects: Mucositis, diarrhea, hand foot syndrome, hyperpigmentation • Activity increased if given with folinic acid. 5 -FU = 5 -fluorouracil 5 -FUR = 5 -fluorouridine 5 -FUMP = 5 -fluorouridine monophosphate 5 -FUDP = 5 -fluorouridine diphosphate 5 -FUTP = 5 -fluorouridine triphosphate d. UMP = deoxyuridine monophosphate d. TMP = deoxythymidine monophosphate 5 -Fd. UMP = 5 -fluorodeoxyuridine monophosphate.

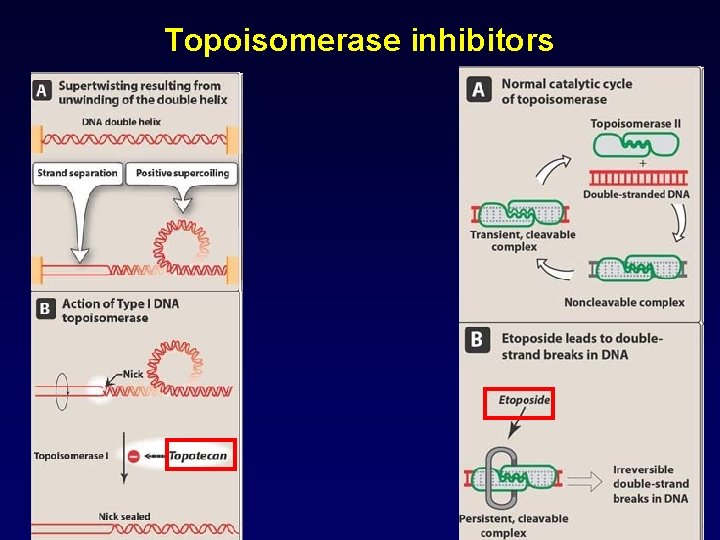
Topoisomerase inhibitors • These drugs are most active in late S and early G 2 phase • Epipodophyllotoxins: Inhibit topoisomerase II – Etoposide – Teniposide • Camptothecins: Inhibit topoisomerase I – Irinotecan – Topotecan

Topoisomerase inhibitors

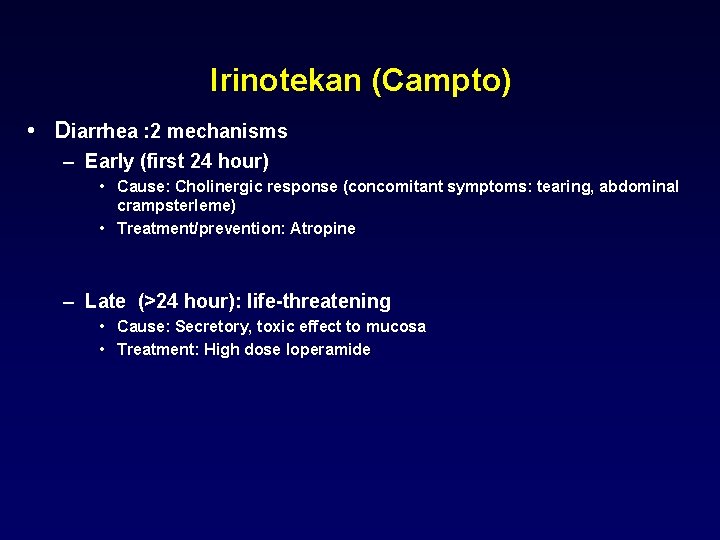
Irinotekan (Campto) • Diarrhea : 2 mechanisms – Early (first 24 hour) • Cause: Cholinergic response (concomitant symptoms: tearing, abdominal crampsterleme) • Treatment/prevention: Atropine – Late (>24 hour): life-threatening • Cause: Secretory, toxic effect to mucosa • Treatment: High dose loperamide

Mitotic spindle inhibitors • Primarily on the M phase of cancer cell cycle • Vinca alkaloids – Vinblastine – Vincristine – Vinorelbine • Taxanes – Paclitaxel – Docetaxel

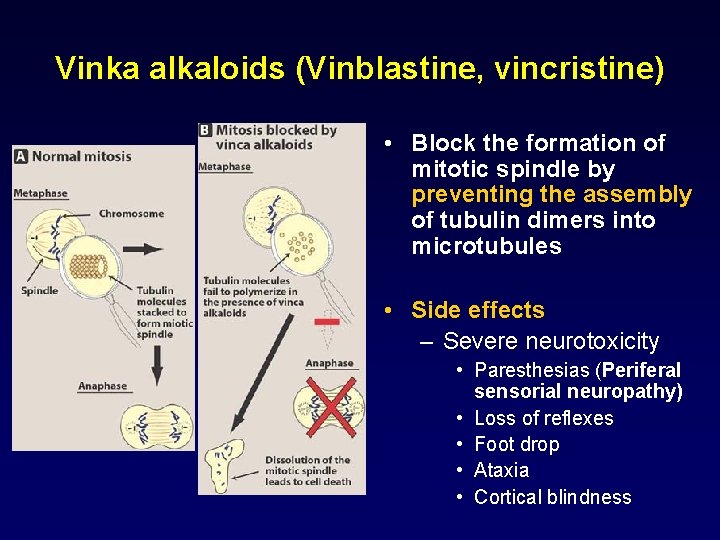
Vinka alkaloids (Vinblastine, vincristine) • Block the formation of mitotic spindle by preventing the assembly of tubulin dimers into microtubules • Side effects – Severe neurotoxicity • Paresthesias (Periferal sensorial neuropathy) • Loss of reflexes • Foot drop • Ataxia • Cortical blindness

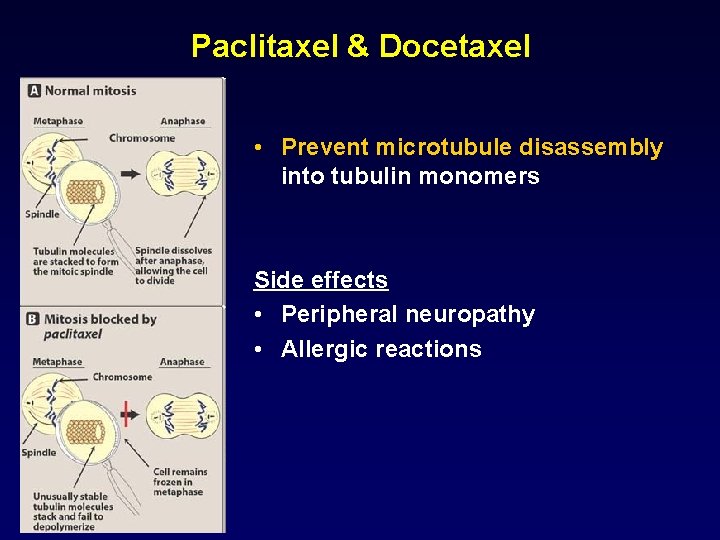
Paclitaxel & Docetaxel • Prevent microtubule disassembly into tubulin monomers Side effects • Peripheral neuropathy • Allergic reactions

Anticancer Antibiotics • Anthracyclines – – Doxorubicin (Adriamycin) Daunorubicin Idarubicin Epirubicin • Bleomycin • Dactinomycin • Mitomycin-C

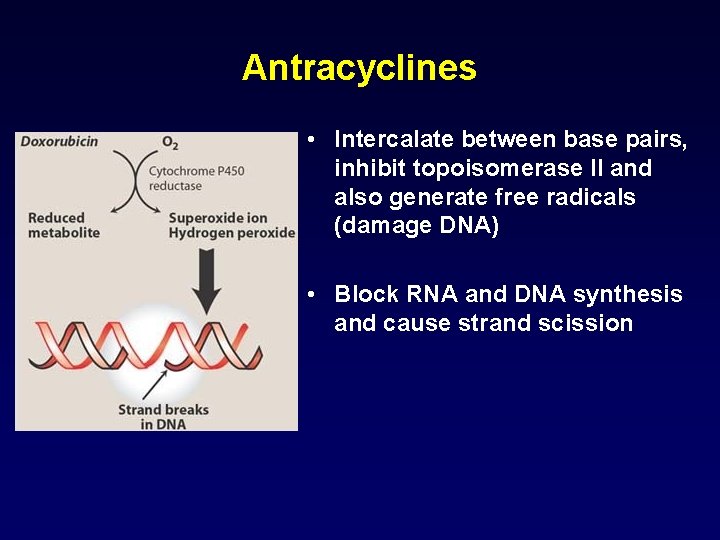
Antracyclines • Intercalate between base pairs, inhibit topoisomerase II and also generate free radicals (damage DNA) • Block RNA and DNA synthesis and cause strand scission

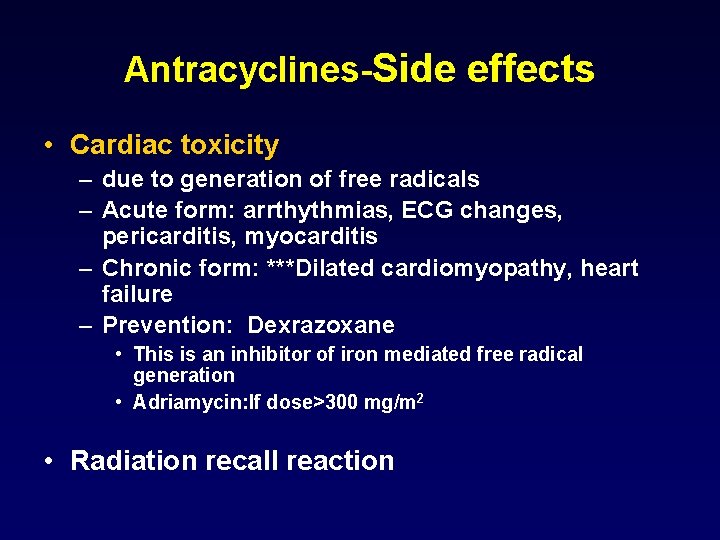
Antracyclines-Side effects • Cardiac toxicity – due to generation of free radicals – Acute form: arrthythmias, ECG changes, pericarditis, myocarditis – Chronic form: ***Dilated cardiomyopathy, heart failure – Prevention: Dexrazoxane • This is an inhibitor of iron mediated free radical generation • Adriamycin: If dose>300 mg/m 2 • Radiation recall reaction

Maximum total dose during lifetime • Adriamycin (Doxorubisin) 450 mg/m 2 • Epirubicine 900 mg/ m 2 • Mitoxantrone 160 mg/m 2 • Bleomycin 200 mg/m 2

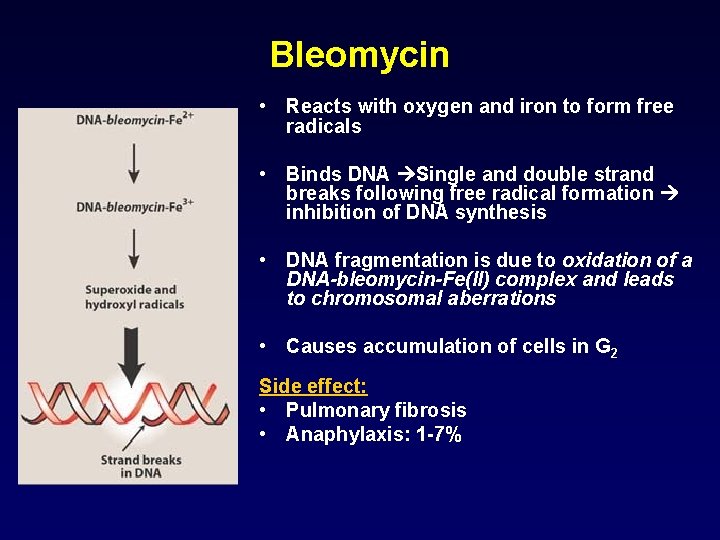
Bleomycin • Reacts with oxygen and iron to form free radicals • Binds DNA Single and double strand breaks following free radical formation inhibition of DNA synthesis • DNA fragmentation is due to oxidation of a DNA-bleomycin-Fe(II) complex and leads to chromosomal aberrations • Causes accumulation of cells in G 2 Side effect: • Pulmonary fibrosis • Anaphylaxis: 1 -7%

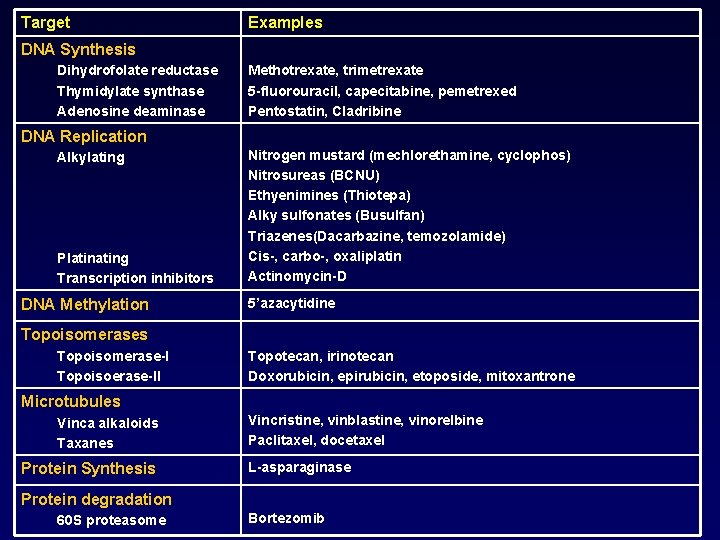
Target Examples DNA Synthesis Dihydrofolate reductase Thymidylate synthase Adenosine deaminase Methotrexate, trimetrexate 5 -fluorouracil, capecitabine, pemetrexed Pentostatin, Cladribine DNA Replication Alkylating Plating Transcription inhibitors DNA Methylation Nitrogen mustard (mechlorethamine, cyclophos) Nitrosureas (BCNU) Ethyenimines (Thiotepa) Alky sulfonates (Busulfan) Triazenes(Dacarbazine, temozolamide) Cis-, carbo-, oxaliplatin Actinomycin-D 5’azacytidine Topoisomerases Topoisomerase-I Topoisoerase-II Topotecan, irinotecan Doxorubicin, epirubicin, etoposide, mitoxantrone Microtubules Vinca alkaloids Taxanes Protein Synthesis Vincristine, vinblastine, vinorelbine Paclitaxel, docetaxel L-asparaginase Protein degradation 60 S proteasome Bortezomib

Extravasation • Vesicant agents: Causes tissue necrosis – Antracyclins (rubicin): Doxorubicin (adriamisin), Daunorubicine, idarubicin – Vinka alkaloids: Vinciristine, vinblastine, vinorelbine, vindesine – Actinomisin-D – Mitomisin – Cisplatin – Nitrojen mustard

Extravasation • İrritant agents: Causes pain, mild inflammation at the injection site or anfd all through vein – – – Mitoxantrone Bleomisin Dacarbazine Carmustine Streptozosin 5 -FU, Etoposide ->seldom • Nonvesicant agents: – L-asparaginaz – Cytarabine – Carboplatin – Cyclophosphamide – Ifosfamide

Side effects of cytotoxic drugs • Anticancer drugs kill fast growing cells – – blood cells progenitors cells in the digestive tract reproductive system hair follicles • Other tissues affected – heart and lungs – kidney and bladder – nerve system

Common toxicities – Neutropenia – Anemia, nausea/vomiting – Diarrhea – Alopecia – Peripheral Neuropathies – Mucositits – Arthralgia/myalgia

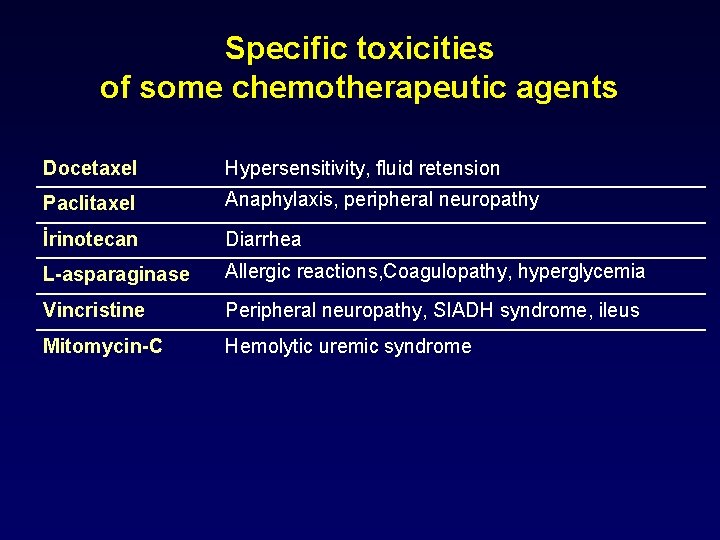
Specific toxicities of some chemotherapeutic agents • Hemorrhagic cystitis • Ifosfamide • Cyclophosphamide • Nephrotoxicity, ototoxicity • Cisplatin • Pulmonary fibrosis • Bleomycin • Busulphan • Allergic reactions • L-asparaginase • Peripheral neuropathy • Cisplatin, Oxaliplatin • Vincristine • Cerebellar dysfunction • Cytarabine • Cardiotoxicity • Antracylines (adriamycim, epirubicine, idarubicin, mitoxantrone)

Specific toxicities of some chemotherapeutic agents Cyclophosphamide Hemorrhagic cystitis, SIADH syndrome Ifosfamide Hemorrhagic cystitis, encephalopathy Cisplatin Nephrotoxicity, ototoxicity, peripheral neuropathy, SIADH syndrome Methotrexate Mucositis, GI ulcers, pulmonary fibrosis, nephrotoxicity, diarrhea 5 -Fluorouracil Stomatitis, GI ulcers, diarrhea, cerebellarataxia, angina Capecitabine Stomatitis, GI ulcers, diarrhea, hand foot syndrome Cytarabine Conjuctivitis, cerebellar dysfunction Antracyclines Cardiotoxicity Bleomycin Pulmonary fibrosis, allergic reactions, anaphylaxis, fever

Specific toxicities of some chemotherapeutic agents Docetaxel Hypersensitivity, fluid retension Paclitaxel Anaphylaxis, peripheral neuropathy İrinotecan Diarrhea L-asparaginase Allergic reactions, Coagulopathy, hyperglycemia Vincristine Peripheral neuropathy, SIADH syndrome, ileus Mitomycin-C Hemolytic uremic syndrome

Late toxicities • Late organ toxicities – – – Heart: Heart failure, MI Lung: Fibrosis Nephrotoxicity Neurotoxicity Immune insufficiency • Secondary malignancies • Early menapouse • Gonadal insufficiency

Principles of combination chemotherapy • Provides maximum cell kill within the range of toxicity tolerated by the host for each drug • Offers a broader range of coverage of resistant cell lines in a heterogeneous tumor population • Prevents or slows the development of new drug resistant cell lines.

Combination chemotherapy Advantages 1. Suppression of drug resistance • less chance of a cell developing resistance to 2 drugs than to 1 drug. 2. Increased cancer cell kill • administration of drugs with different mechanisms of action. 3. Reduced injury to normal cells • by using a combination of drugs that do not have overlapping toxicities, we can achieve a greater anticancer effect than we could by using any one agent alone.

Combination chemotherapy • Agents with single agent activity • Different mechanism of actions • Different toxicities

Aim of combination therapy INCREASED EFFICACY ACTIVITY Different mechanisms of action Different mechanisms of resistance SAFETY Compatible side effects

Drug resistance • Primary resistance • Acquired resistance • • • Decreased intracellular uptake Increased efflux Change in target of drug Increased DNA repair Metabolic changes Gen amplification in target zone

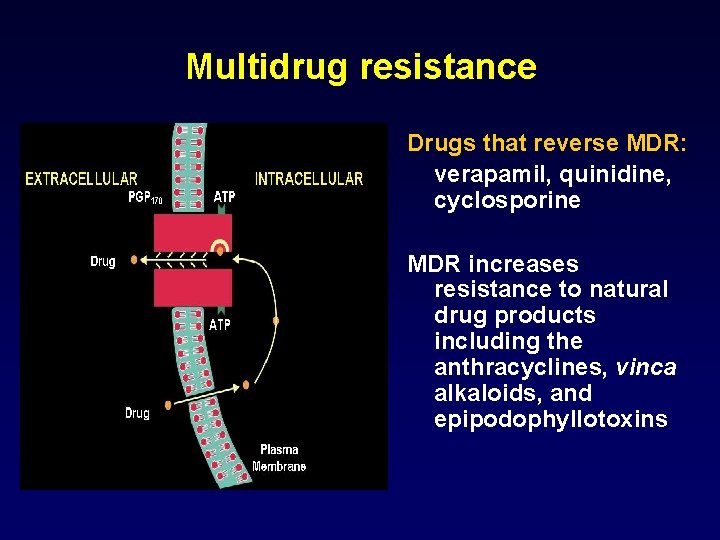
Multidrug resistance Drugs that reverse MDR: verapamil, quinidine, cyclosporine MDR increases resistance to natural drug products including the anthracyclines, vinca alkaloids, and epipodophyllotoxins

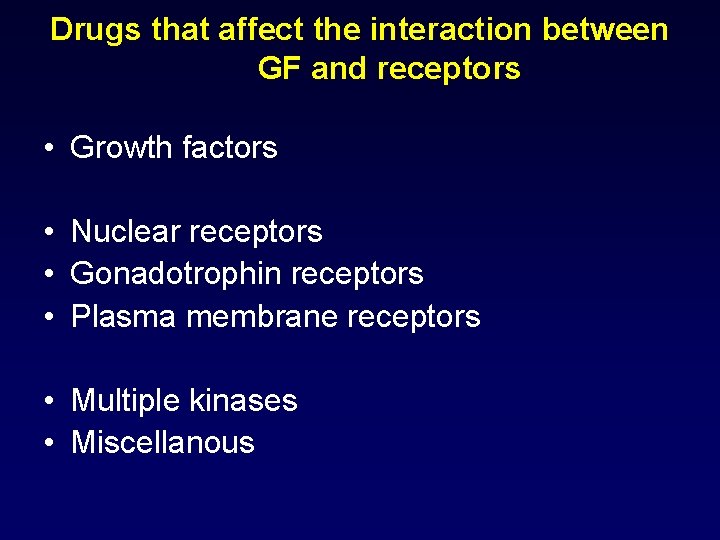
Drugs that affect the interaction between GF and receptors • Growth factors • Nuclear receptors • Gonadotrophin receptors • Plasma membrane receptors • Multiple kinases • Miscellanous


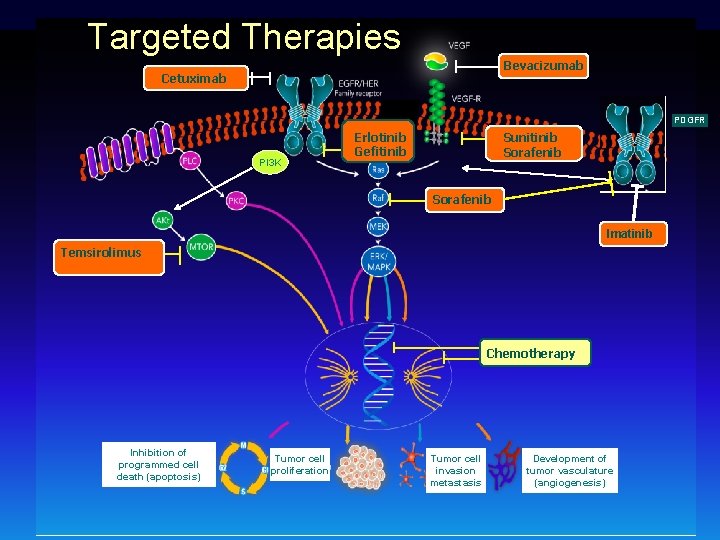
Target Monoclonal antibody Tyrosine kinase inhibitor VEGF Bevacizumab - VEGFR - Sunitinib Angiogenesis Sorafenib EGFR Cetuximab Erlotinib Gefitinib Her 2 Trastuzumab Bcr-abl CD 20 Lapatinib İmatinib Rituximab

Targeted Therapies Bevacizumab Cetuximab PDGFR PI 3 K Erlotinib Gefitinib Sunitinib Sorafenib Imatinib Temsirolimus Chemotherapy Inhibition of programmed cell death (apoptosis) Tumor cell proliferation Tumor cell invasion metastasis Development of tumor vasculature (angiogenesis)

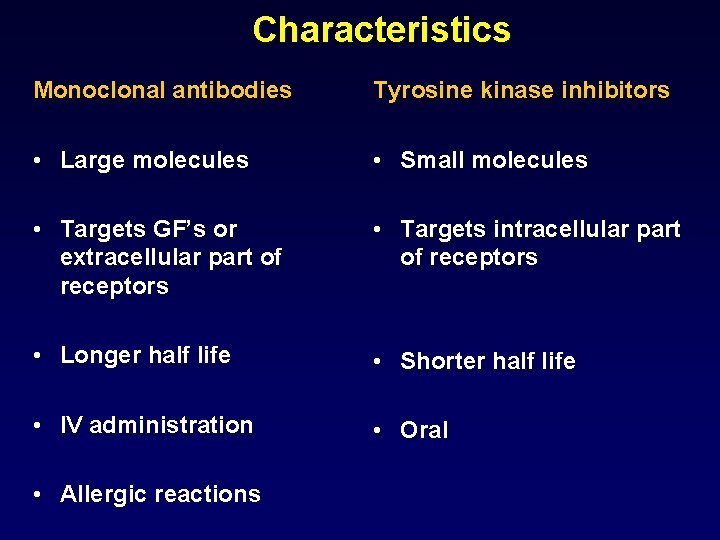
Characteristics Monoclonal antibodies Tyrosine kinase inhibitors • Large molecules • Small molecules • Targets GF’s or extracellular part of receptors • Targets intracellular part of receptors • Longer half life • Shorter half life • IV administration • Oral • Allergic reactions

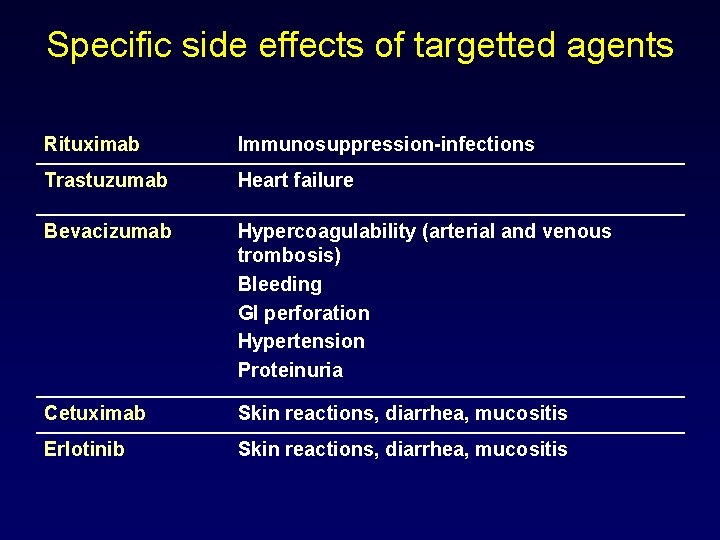
Specific side effects of targetted agents Rituximab Immunosuppression-infections Trastuzumab Heart failure Bevacizumab Hypercoagulability (arterial and venous trombosis) Bleeding GI perforation Hypertension Proteinuria Cetuximab Skin reactions, diarrhea, mucositis Erlotinib Skin reactions, diarrhea, mucositis

Thank you
 Principles of chemotherapy
Principles of chemotherapy General principles of chemotherapy
General principles of chemotherapy Antracyclins
Antracyclins Types of housekeeping
Types of housekeeping Bsa calculation formula for chemotherapy
Bsa calculation formula for chemotherapy Icd 9 code for aphthous ulcer
Icd 9 code for aphthous ulcer Chemotherapy
Chemotherapy Chemotherapy
Chemotherapy Early help assessment training
Early help assessment training 4ac 4t chemotherapy
4ac 4t chemotherapy Complications of blood transfusion
Complications of blood transfusion Blood transfusion complications
Blood transfusion complications What's a short story
What's a short story Elements of a setting
Elements of a setting Thyroid
Thyroid Eswl complications
Eswl complications Complications c section
Complications c section Sleeve gastrectomy complications
Sleeve gastrectomy complications Complications of shigellosis
Complications of shigellosis Trachestomy tube
Trachestomy tube Bronchial asthma
Bronchial asthma Who stroke definition
Who stroke definition Thoracentesis complications
Thoracentesis complications Eclampsia definicion
Eclampsia definicion Bishop score
Bishop score Post dated pregnancy complications
Post dated pregnancy complications عکس برش پرینه در زایمان طبیعی
عکس برش پرینه در زایمان طبیعی Blood transfusion complications
Blood transfusion complications Bronchoscopy complications
Bronchoscopy complications Neph tubes
Neph tubes How to mix shingrix vaccine
How to mix shingrix vaccine Bronchoscopy complications
Bronchoscopy complications Dr. tachere
Dr. tachere Kesselbachs plexus
Kesselbachs plexus Dialysis purpose
Dialysis purpose Phlegmasia cerulea dolens definition
Phlegmasia cerulea dolens definition Complication of liver cirrhosis
Complication of liver cirrhosis Abdominal insufflation complications
Abdominal insufflation complications Iliac stent complications
Iliac stent complications Heart failure complications
Heart failure complications Ng tube types
Ng tube types Complications of gestational diabetes
Complications of gestational diabetes C section layers
C section layers Central venous line complications
Central venous line complications Doyen retractor contraindications
Doyen retractor contraindications C section complications
C section complications Complication of blood transfusion
Complication of blood transfusion Complications of blood transfusion
Complications of blood transfusion Hyperlipidemia complications
Hyperlipidemia complications Complications of anemia
Complications of anemia Complications of blood transfusion
Complications of blood transfusion Magnesium sulfate indication
Magnesium sulfate indication Complications of lupus
Complications of lupus Septic arthritis complications
Septic arthritis complications Glomerulonephritis complications
Glomerulonephritis complications Walid radwan
Walid radwan Brain hemorrhage
Brain hemorrhage Complications of hydrocephalus
Complications of hydrocephalus Complications of hydrocephalus
Complications of hydrocephalus What are the complications of blood transfusion
What are the complications of blood transfusion Complications of bacterial meningitis
Complications of bacterial meningitis Septic arthritis complications
Septic arthritis complications Sumeet garg
Sumeet garg 15yo
15yo Complication of iv infusion
Complication of iv infusion Slab in orthopaedics
Slab in orthopaedics Colostomy indications and contraindications
Colostomy indications and contraindications Gastrostomie complications
Gastrostomie complications Internal vs external fixation fracture
Internal vs external fixation fracture Complications of myelofibrosis
Complications of myelofibrosis Pathophysiology of hypothyroidism
Pathophysiology of hypothyroidism Centrifuged
Centrifuged Otitis media complications
Otitis media complications Complications of local anesthesia in dentistry
Complications of local anesthesia in dentistry