Principles and Practice of Cleaning Sterility in Place




















- Slides: 36

Principles and Practice of Cleaning & Sterility in Place

What is CIP? CIP or in its full form, Cleaning in Place is defined as: Equipment and techniques to allow cleaning or process equipment without dismantling or manual cleaning with minimal operator involvement.

CIP / SIP - Definition • CIP = Cleaning in Place – To clean the product contact surfaces of: • vessels, • equipment and • pipework – in place – i. e. without dismantling • SIP = Sterilize in Place – To ensure product contact surfaces are sufficiently sterile to minimise product infection.

CIP / SIP • Involves the use of: – Chemicals – Sterilizers – High pressure pumps – Spray nozzles – Spray balls – Aseptic designs

CIP or SIP • Ensures that the large scale processes are: – Free of dirt – Free of organic contaminants – Free of microorganisms

Benefits of CIP

Benefits, contd. • It is: – Automatic – Reproducible – Reliable • Delivery of: – Cleaning solutions – Rinsing – Washing • To & through: – process equipment/piping • Improves: – Product quality – Plant hygiene

How CIP Works? • Mechanical – Removes ‘loose’ particles by • Impact / Turbulence • Chemical – Breaks up /removes remaining particles, if possible, by • Chemical action • Sterilant/Sanitizer – ‘Kills’ remaining micro-organisms • to an acceptable level

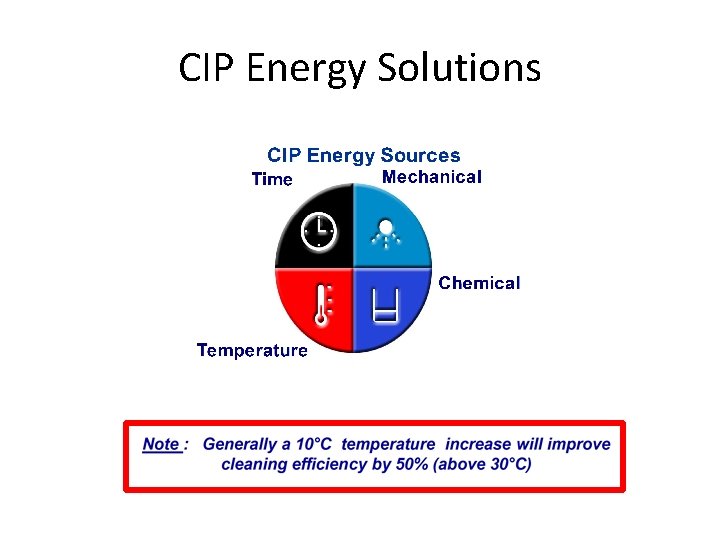
CIP Energy Solutions

Factors affecting CIP • Mechanical • Chemical • Temperature • Time

CIP Operation • PRE-RINSE - Mechanical Removal • DETERGENT - Cleaning of Remaining - Caustic, Acid or Both • FINAL RINSE - Wash Residual Detergent • STERILANT/SANITIZER - Cold or Hot

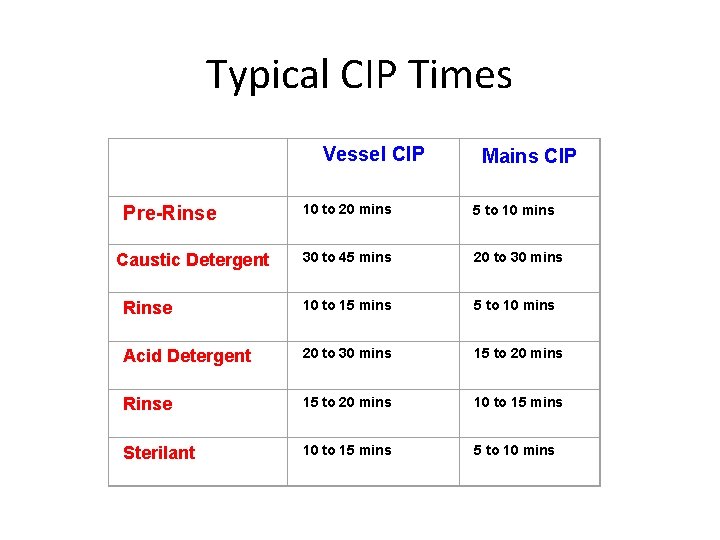
Typical CIP Times Vessel CIP Mains CIP 10 to 20 mins 5 to 10 mins 30 to 45 mins 20 to 30 mins Rinse 10 to 15 mins 5 to 10 mins Acid Detergent 20 to 30 mins 15 to 20 mins Rinse 15 to 20 mins 10 to 15 mins Sterilant 10 to 15 mins 5 to 10 mins Pre-Rinse Caustic Detergent

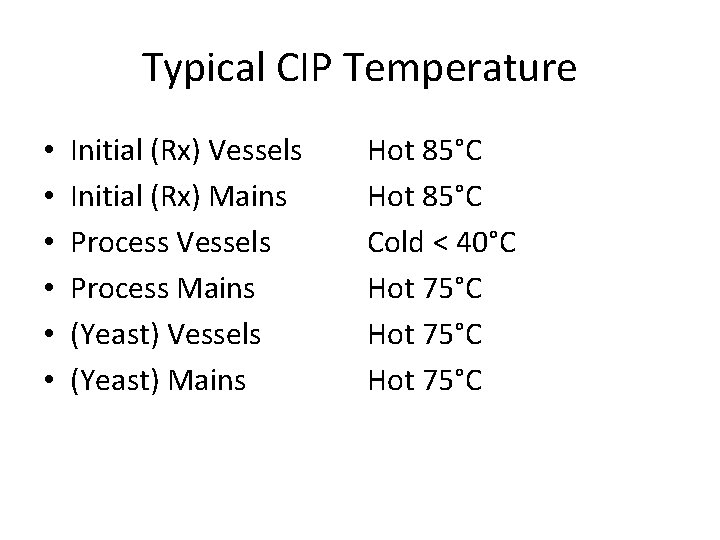
Typical CIP Temperature • • • Initial (Rx) Vessels Initial (Rx) Mains Process Vessels Process Mains (Yeast) Vessels (Yeast) Mains Hot 85°C Cold < 40°C Hot 75°C

Water Used for Cleaning Process • Quality of Water used for aqueous cleansing is critical for performance: • Chemical properties (p. H, hardness, etc. ) • Biological properties (bioburden, endotoxins) • Pre-Rinsing. Solely for flushing out of residue prior to washing step. Usually based on practicality of what water is available. • Washing. Most critical water hardness – effects efficiency of cleansing of aqueous surfactant solutions. • Rinsing. In general, the final rinse used for equipment should use the same quality of water as used in the final stage of manufacture.

CIP Detergent Requirements • • • Effective on target Non foaming or include anti-foam Free rinsing Non corrosive – Vessels/pipes, joints Controllable - Conductivity Environmental

Caustic Detergents • Advantages – Excellent detergency properties when “formulated” – Disinfection properties, especially when used hot. – Effective at removal of protein – Auto strength control by conductivity meter – More effective than acid – Cost effective • Disadvantages – Degraded by CO 2 forming carbonate. – Ineffective at removing inorganic scale. – Poor ability to rinse. – Not compatible with Aluminium – Activity affected by water hardness.

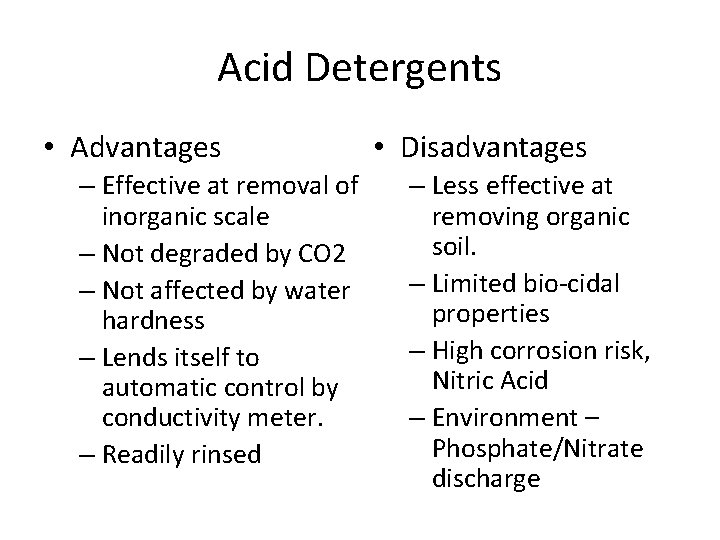
Acid Detergents • Advantages – Effective at removal of inorganic scale – Not degraded by CO 2 – Not affected by water hardness – Lends itself to automatic control by conductivity meter. – Readily rinsed • Disadvantages – Less effective at removing organic soil. – Limited bio-cidal properties – High corrosion risk, Nitric Acid – Environment – Phosphate/Nitrate discharge

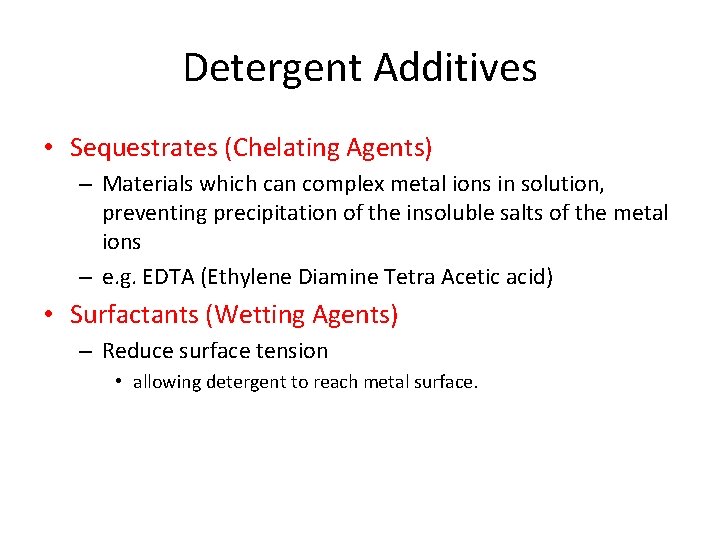
Detergent Additives • Sequestrates (Chelating Agents) – Materials which can complex metal ions in solution, preventing precipitation of the insoluble salts of the metal ions – e. g. EDTA (Ethylene Diamine Tetra Acetic acid) • Surfactants (Wetting Agents) – Reduce surface tension • allowing detergent to reach metal surface.

Sterilant / Sanitizer Requirements • • • Effective against target organisms Fast Acting Low Hazard Low Corrosion Non Tainting (An unpleasant odour and flavor) Acceptable Foam Characteristics

Sterilants / Sanitizers • • Chlorine Dioxide Hypochlorite Iodophor Acid Anionic Quaternary Ammonium Hydrogen Peroxide PAA (Per-oxy Acetic Acid)

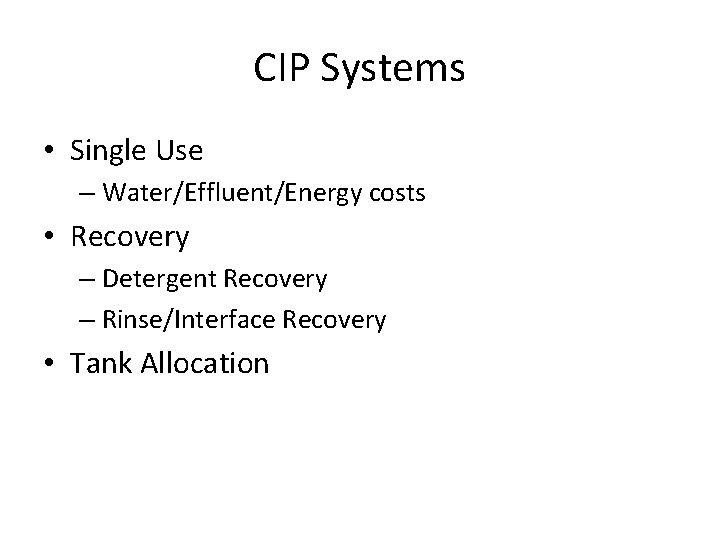
CIP Systems • Single Use – Water/Effluent/Energy costs • Recovery – Detergent Recovery – Rinse/Interface Recovery • Tank Allocation

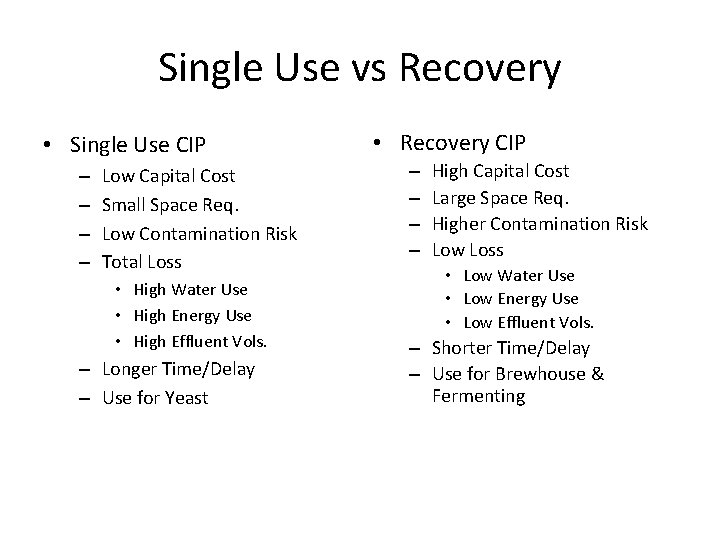
Single Use vs Recovery • Single Use CIP – – Low Capital Cost Small Space Req. Low Contamination Risk Total Loss • High Water Use • High Energy Use • High Effluent Vols. – Longer Time/Delay – Use for Yeast • Recovery CIP – – High Capital Cost Large Space Req. Higher Contamination Risk Low Loss • Low Water Use • Low Energy Use • Low Effluent Vols. – Shorter Time/Delay – Use for Brewhouse & Fermenting

Types of CIP • VESSEL CIP - Spray-head Selection - Scavenge Control • MAINS CIP - Adequate Velocity - Total Route Coverage

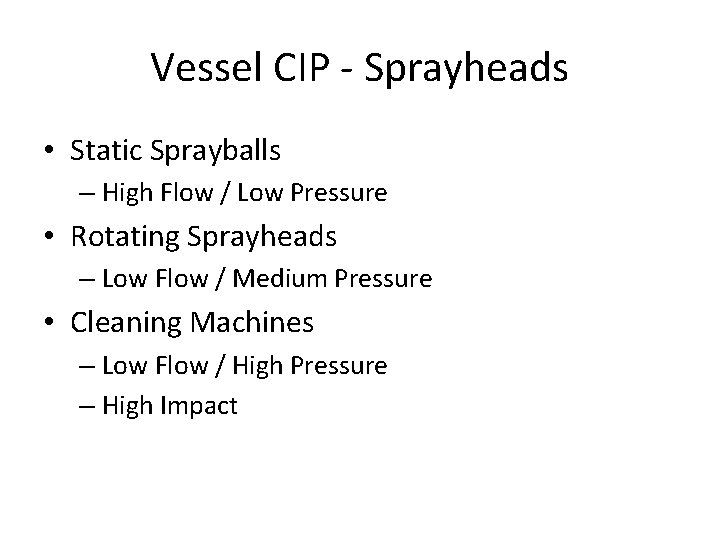
Vessel CIP - Sprayheads • Static Sprayballs – High Flow / Low Pressure • Rotating Sprayheads – Low Flow / Medium Pressure • Cleaning Machines – Low Flow / High Pressure – High Impact

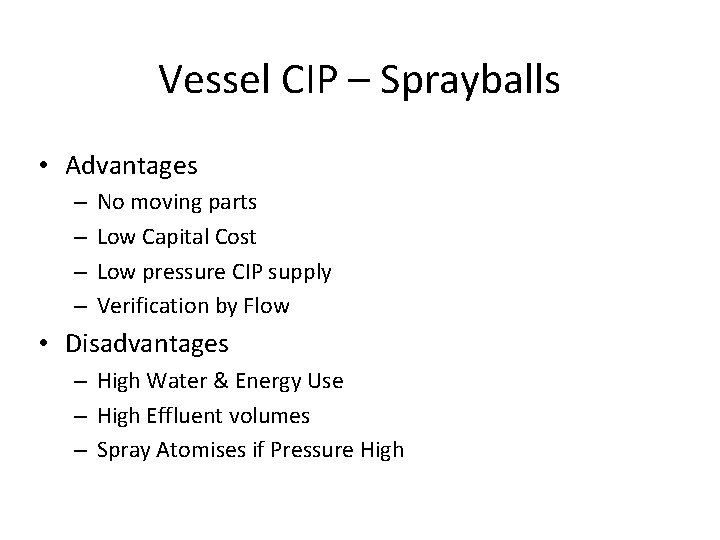
Vessel CIP – Sprayballs • Advantages – – No moving parts Low Capital Cost Low pressure CIP supply Verification by Flow • Disadvantages – High Water & Energy Use – High Effluent volumes – Spray Atomises if Pressure High

Vessel CIP – Cleaning Machines • Advantages – High impact, aggressive cleaning – Good for heavy duty cleaning – Low water/energy use – Low effluent – Effective in large vessels

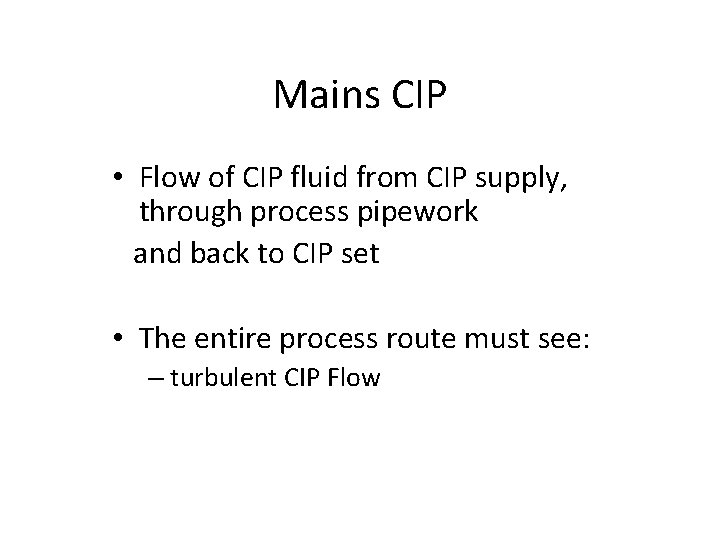
Mains CIP • Flow of CIP fluid from CIP supply, through process pipework and back to CIP set • The entire process route must see: – turbulent CIP Flow

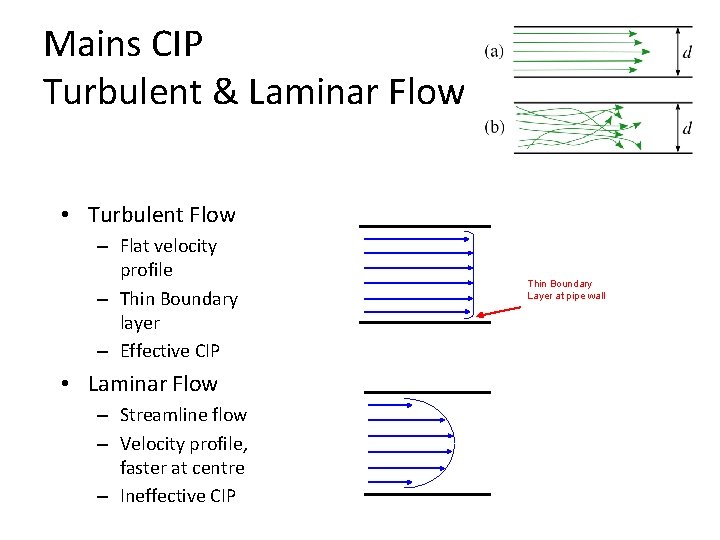
Mains CIP Turbulent & Laminar Flow • Turbulent Flow – Flat velocity profile – Thin Boundary layer – Effective CIP • Laminar Flow – Streamline flow – Velocity profile, faster at centre – Ineffective CIP Thin Boundary Layer at pipe wall

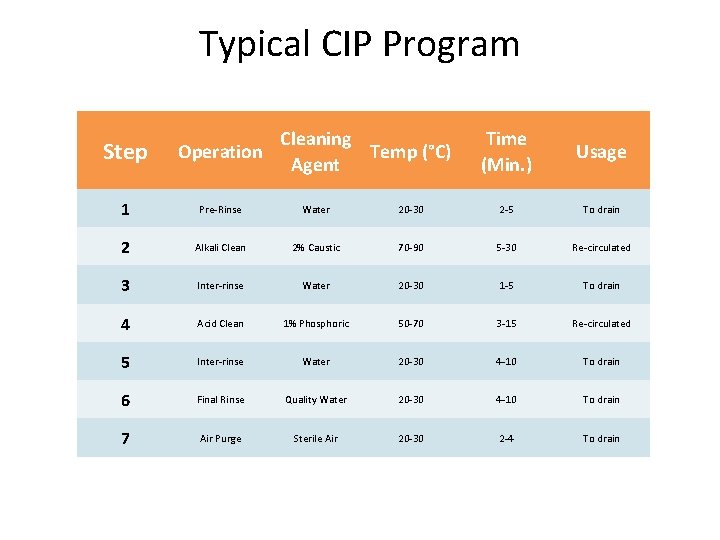
Typical CIP Program Cleaning Temp (°C) Agent Time (Min. ) Usage 20 -30 2 -5 To drain 2% Caustic 70 -90 5 -30 Re-circulated Inter-rinse Water 20 -30 1 -5 To drain 4 Acid Clean 1% Phosphoric 50 -70 3 -15 Re-circulated 5 Inter-rinse Water 20 -30 4 -10 To drain 6 Final Rinse Quality Water 20 -30 4 -10 To drain 7 Air Purge Sterile Air 20 -30 2 -4 To drain Step Operation 1 Pre-Rinse Water 2 Alkali Clean 3

Why Use CIP? • 1. CIP is superior to any cleaning method Automated, with parameter monitoring & control Repeatablility -> reliability Human errors eliminated Eliminate contaminated products

Why Use CIP? (pt. 2) • 2) Lower operating costs Reduced labor costs Cleaning turnaround time reduced Water / solvents / detergents usage significantly reduced

Why Use CIP? (pt. 3) • 3) Safety Improvement Reduced explosive of product to personnel No equipment dismantling / vessel entry Eliminates hazardous activities, eg HP water blasting

What is the Result of CIP? • CIP results in the equipment being chemically cleaned. This is defined as “the removal of all residues of soil and all CIP agents so that contact with the cleaned surface does not result in physical contamination. ” • If the equipment being cleaned needs to be microbiologically clean then an additional process can be carried out. This process is called SIP.

What is SIP? • SIP or in its full form, Sterlilizing in place is the generic term for sanitizing, disinfecting or sterilizing equipment normally after a CIP clean. • SIP results in the removal of any remaining microbiological contamination.

Chemical SIP • Sanitation or Disinfection is normally applied after the full CIP has been carried out. It is achieved by the introduction of a sanitizer or disinfectant chemical into the final rinse waters of the CIP Typical Chemical Sanitizers are: Chlorine, hypochlorite, hydrogen peroxide, ozone, peracetic acid

Thermal SIP Thermal sterilization has the advantage of affecting areas such as sample points, which may not be treated by chemical means. Thermal sterilization is achieved by the application of steam or hot water at a suitable temperature for a suitable firm Typical Thermal SIP methods are: dry heart, steam, superheated water.