Principle of PCR Prof Dr Baron 1 PCR

Principle of PCR Prof. Dr. Baron 1
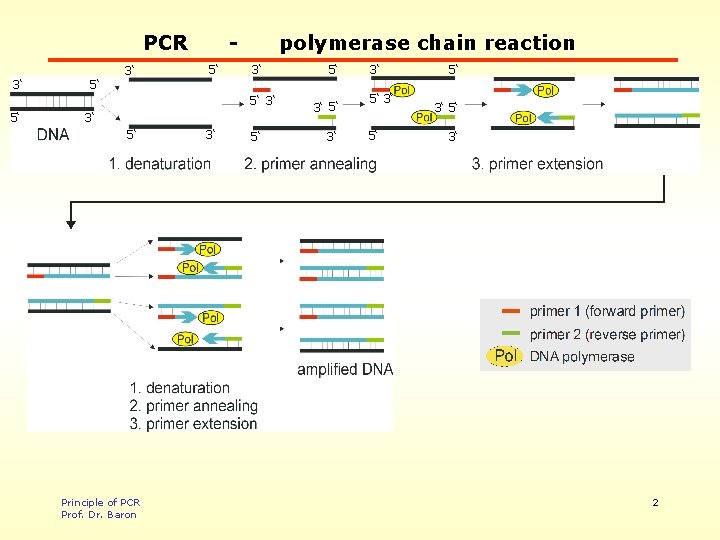
PCR 3‘ 3‘ 5‘ polymerase chain reaction 3‘ 5‘ 5‘ 3‘ 5‘ Principle of PCR Prof. Dr. Baron 3‘ 5‘ 3‘ 3‘ 5‘ 5‘ 3‘ 2
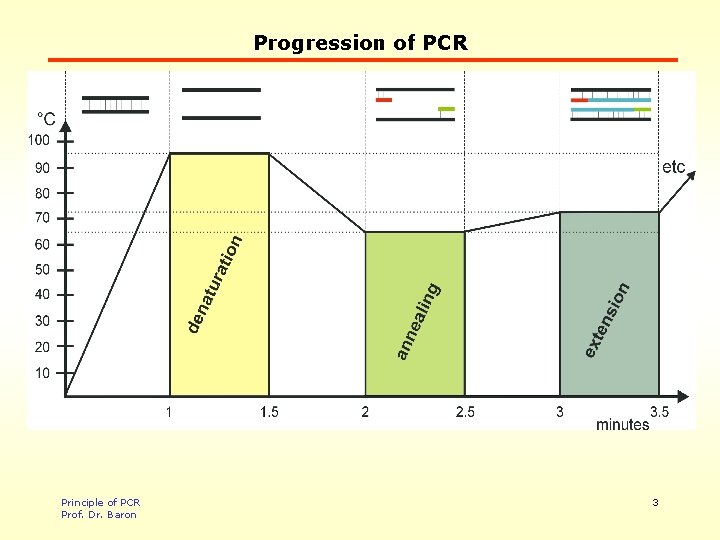
Progression of PCR Principle of PCR Prof. Dr. Baron 3
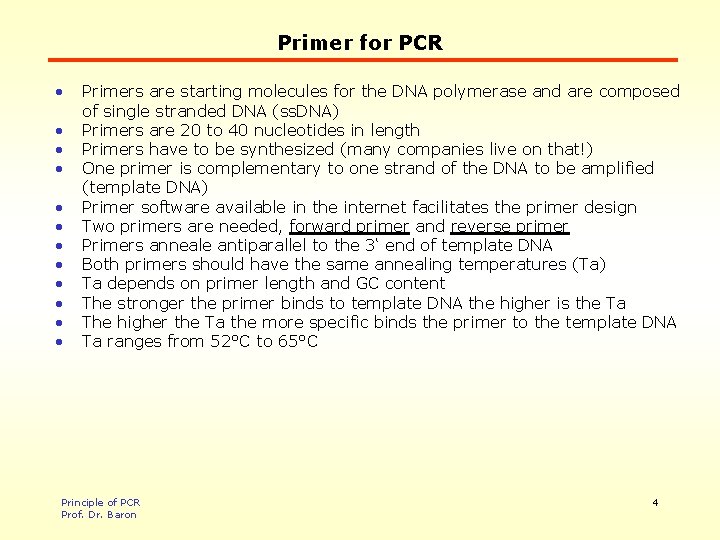
Primer for PCR • • • Primers are starting molecules for the DNA polymerase and are composed of single stranded DNA (ss. DNA) Primers are 20 to 40 nucleotides in length Primers have to be synthesized (many companies live on that!) One primer is complementary to one strand of the DNA to be amplified (template DNA) Primer software available in the internet facilitates the primer design Two primers are needed, forward primer and reverse primer Primers anneale antiparallel to the 3‘ end of template DNA Both primers should have the same annealing temperatures (Ta) Ta depends on primer length and GC content The stronger the primer binds to template DNA the higher is the Ta The higher the Ta the more specific binds the primer to the template DNA Ta ranges from 52°C to 65°C Principle of PCR Prof. Dr. Baron 4

PCR of 20 Cycles Principle of PCR Prof. Dr. Baron 5
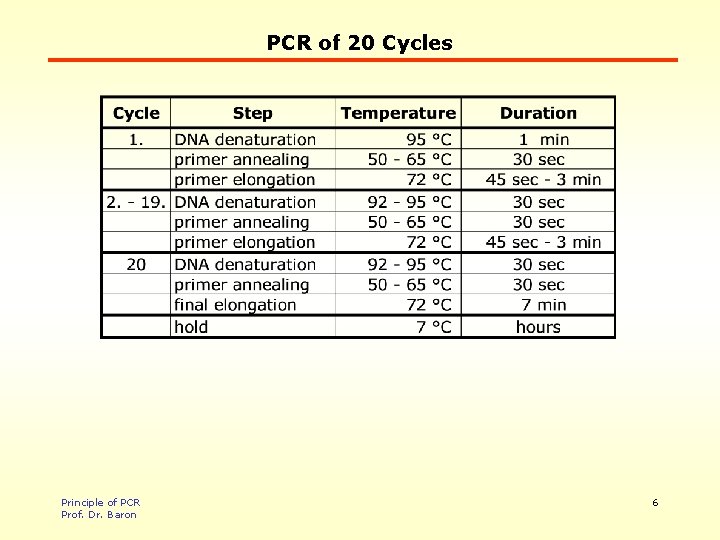
PCR of 20 Cycles Principle of PCR Prof. Dr. Baron 6
Amplification by PCR of DNA • • • Amplification from 20 to 50 cycles - 20 cycles 220 copies of DNA = 106 -fold amplification - 30 cycles 230 copies of DNA = 109 -fold amplification - 40 cycles 240 copies of DNA = 1012 -fold amplification Formula: 2 n DNA copies, n = number of PCR cycles Elaborated by K. Mullis in 1985, nobel price in 1993, use of DNA polymerase from E. coli, no automation possible Automation was possible after introducing heat stabile DNA polymerases (e. g. Taq-Polymerase) Automation of PCR by programmable thermal cycler 1 st cycle yields DNA of undefined length, two length-defined DNA copies after the 3 rd cycle Principle of PCR Prof. Dr. Baron 7
Taq-Polymerase • • • origin/organism Thermus aquaticus habitat geyser, Yellowstone NP ('76) molecular weight 94 k. Da (rec. from E. coli) p. H optimum 7– 8 temperature optimum in vitro 70 - 80°C rate of synthesis 150 nucleotides/s @ 75 -80°C 22 nucleotides/s @ 55°C 1. 5 nucleotides/s @ 37°C 0. 25 nucleotides/s @ 22°C error rate in vitro 1 in 5000 nucleotides stability/biological half life 12 min @ 94°C 6 min @ 97°C additional enzyme activity 5´ 3´ exonuclease limitation of numbers of PCR cycles • biological half life of Taq polymerase @ 95°C • shortage of primers and d. NTP's • amplified ds. DNA binds primer Principle of PCR Prof. Dr. Baron 8
End Principle of PCR Prof. Dr. Baron 9
- Slides: 9