Primitive and conventional cells StructureLatticebasis Primitive cell parallelepiped
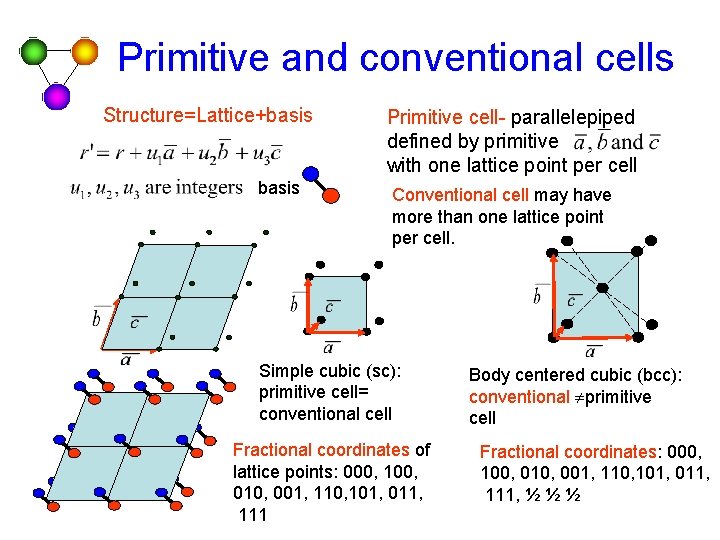
Primitive and conventional cells Structure=Lattice+basis Primitive cell- parallelepiped defined by primitive with one lattice point per cell Conventional cell may have more than one lattice point per cell. Simple cubic (sc): primitive cell= conventional cell Fractional coordinates of lattice points: 000, 100, 010, 001, 110, 101, 011, 111 Body centered cubic (bcc): conventional ¹primitive cell Fractional coordinates: 000, 100, 010, 001, 110, 101, 011, 111, ½ ½ ½
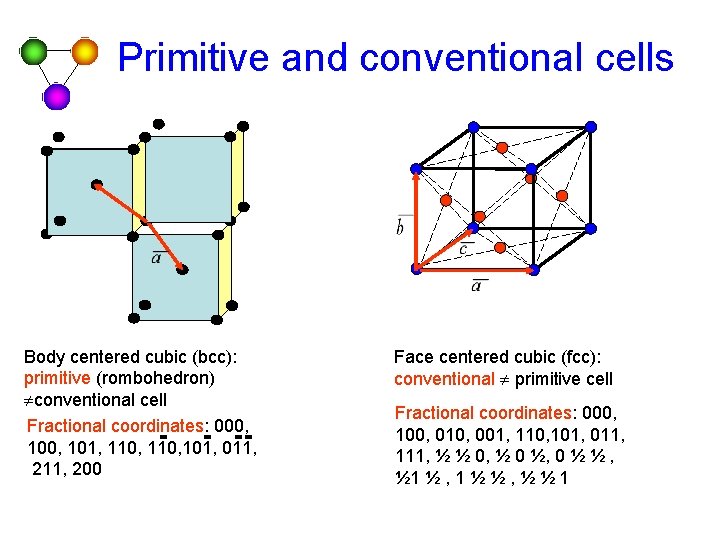
Primitive and conventional cells Body centered cubic (bcc): primitive (rombohedron) ¹conventional cell Fractional coordinates: 000, 101, 110, 101, 011, 200 Face centered cubic (fcc): conventional ¹ primitive cell Fractional coordinates: 000, 100, 010, 001, 110, 101, 011, 111, ½ ½ 0, ½ 0 ½, 0 ½ ½ , ½ 1 ½ , 1 ½ ½ , ½ ½ 1
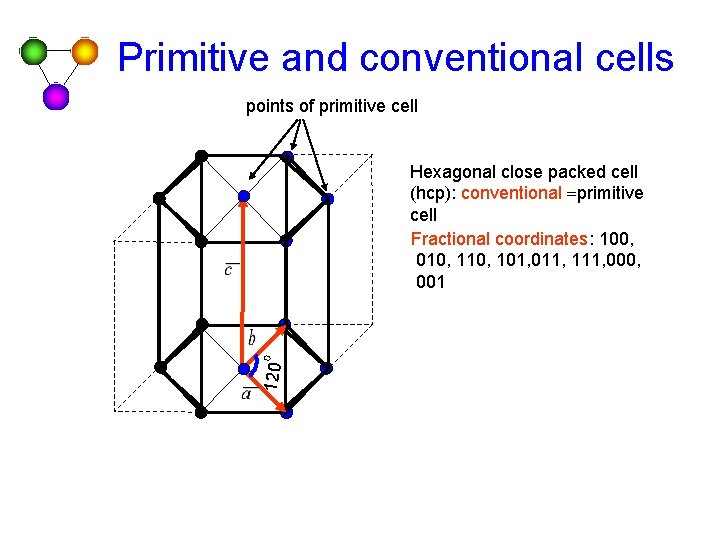
Primitive and conventional cells points of primitive cell 120 o Hexagonal close packed cell (hcp): conventional =primitive cell Fractional coordinates: 100, 010, 101, 011, 111, 000, 001
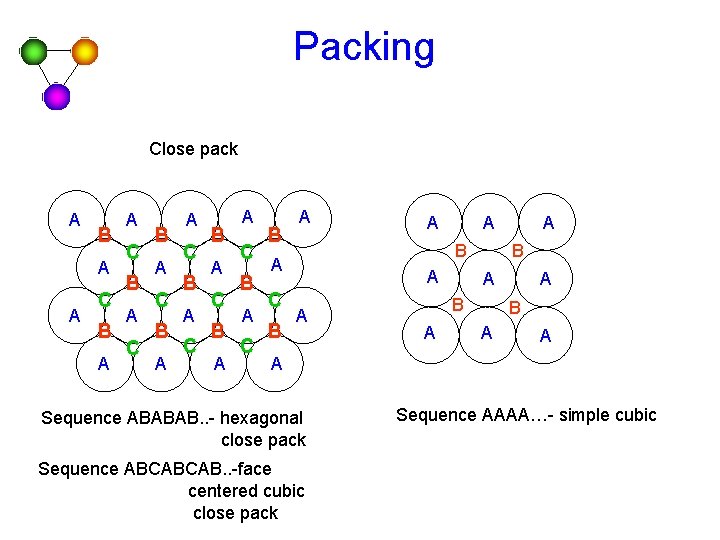
Packing Close pack A B A A C B A C B A C B A B A C A A A B A A A Sequence ABABAB. . - hexagonal close pack Sequence ABCABCAB. . -face centered cubic close pack Sequence AAAA…- simple cubic
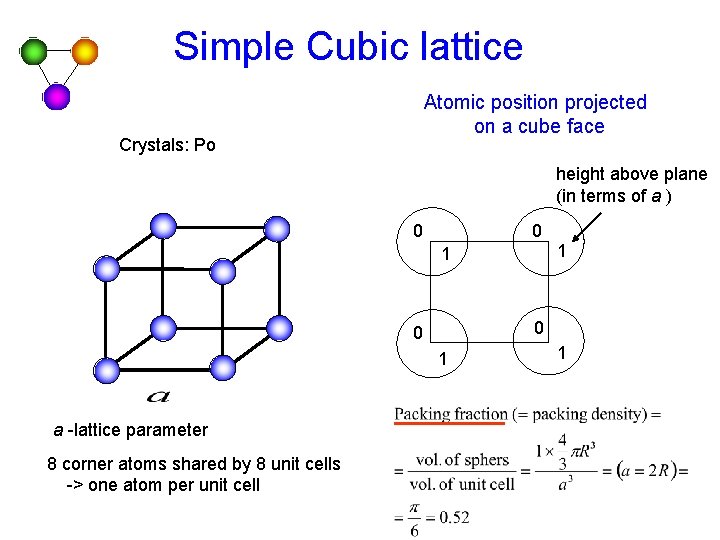
Simple Cubic lattice Atomic position projected on a cube face Crystals: Po height above plane (in terms of a ) 0 0 1 a -lattice parameter 8 corner atoms shared by 8 unit cells -> one atom per unit cell 1 1
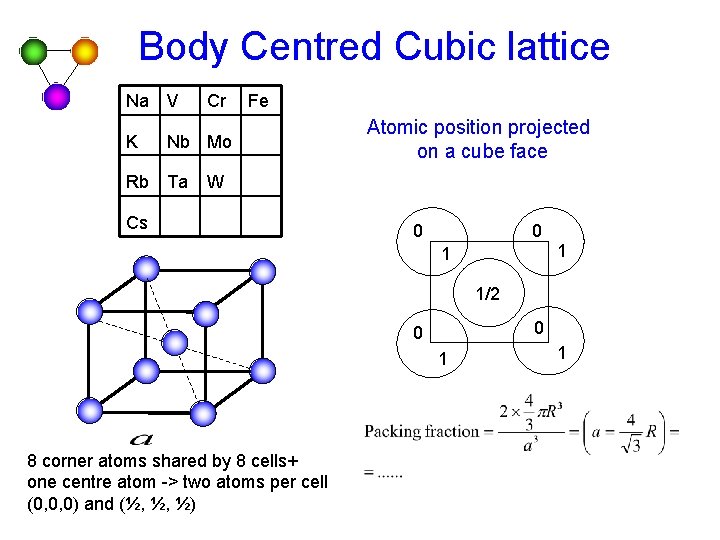
Body Centred Cubic lattice Na V K Cr Fe Nb Mo Rb Ta Atomic position projected on a cube face W Cs 0 0 1 1 1/2 0 0 1 8 corner atoms shared by 8 cells+ one centre atom -> two atoms per cell (0, 0, 0) and (½, ½, ½) 1
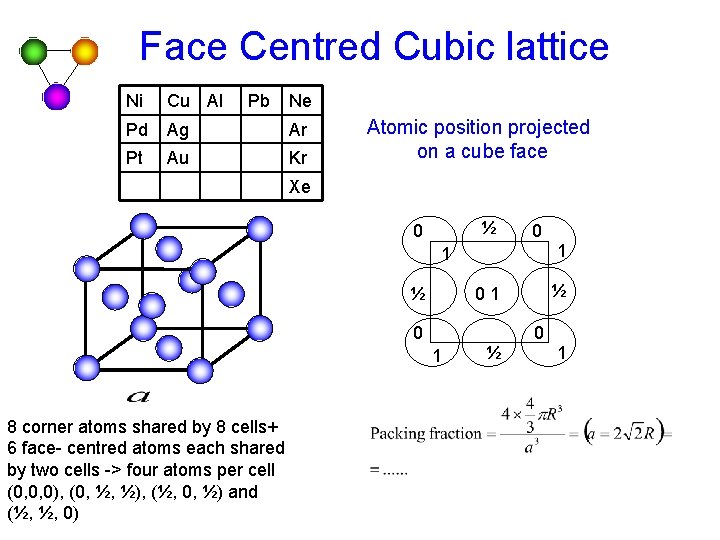
Face Centred Cubic lattice Ni Cu Al Pb Ne Pd Ag Ar Pt Au Kr Atomic position projected on a cube face Xe ½ 0 0 1 ½ 1 8 corner atoms shared by 8 cells+ 6 face- centred atoms each shared by two cells -> four atoms per cell (0, 0, 0), (0, ½, ½), (½, 0, ½) and (½, ½, 0) ½ 01 0 ½ 1 0 1
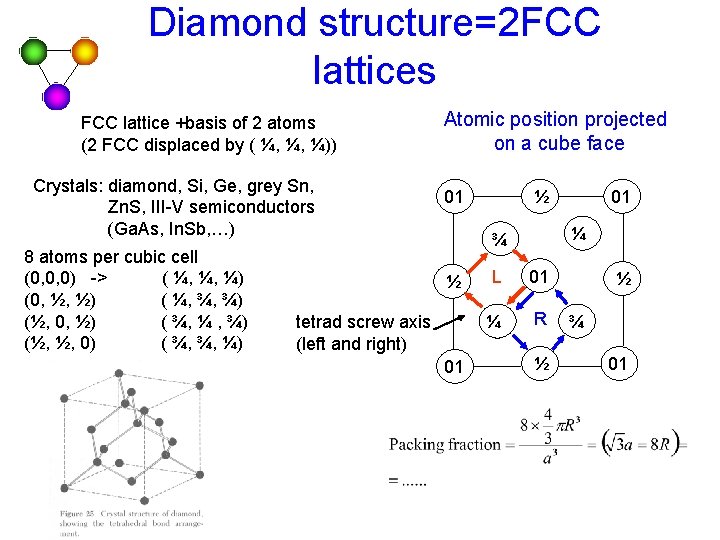
Diamond structure=2 FCC lattices FCC lattice +basis of 2 atoms (2 FCC displaced by ( ¼, ¼, ¼)) Crystals: diamond, Si, Ge, grey Sn, Zn. S, III-V semiconductors (Ga. As, In. Sb, …) 8 atoms per cubic cell (0, 0, 0) -> ( ¼, ¼, ¼) (0, ½, ½) ( ¼, ¾, ¾) (½, 0, ½) ( ¾, ¼ , ¾) (½, ½, 0) ( ¾, ¾, ¼) Atomic position projected on a cube face 01 ½ ¼ ¾ ½ tetrad screw axis (left and right) 01 01 L 01 ¼ R ½ ½ ¾ 01
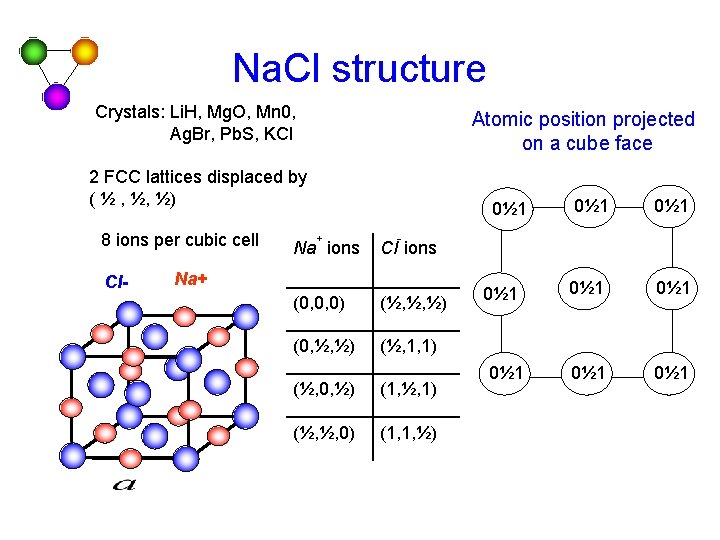
Na. Cl structure Crystals: Li. H, Mg. O, Mn 0, Ag. Br, Pb. S, KCl Atomic position projected on a cube face 2 FCC lattices displaced by ( ½ , ½, ½) 8 ions per cubic cell Cl- 0½ 1 + Na ions 0½ 1 - Cl ions Na+ (0, 0, 0) (½, ½, ½) (0, ½, ½) (½, 1, 1) (½, 0, ½) (1, ½, 1) (½, ½, 0) (1, 1, ½) 0½ 1 0½ 1
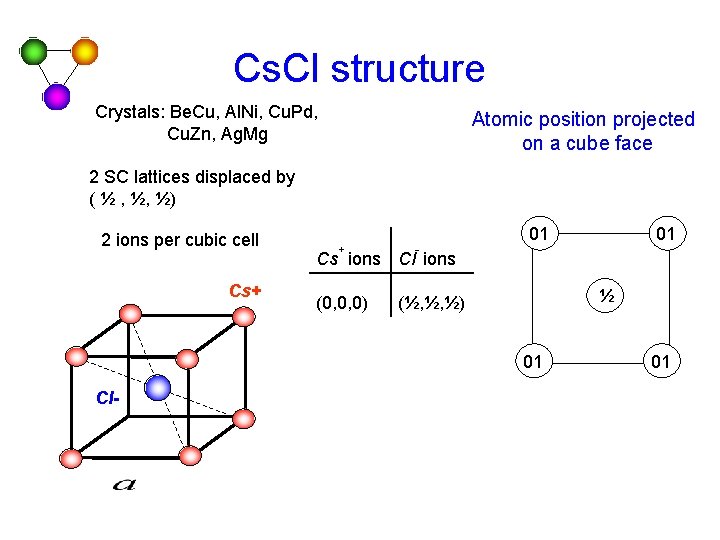
Cs. Cl structure Crystals: Be. Cu, Al. Ni, Cu. Pd, Cu. Zn, Ag. Mg Atomic position projected on a cube face 2 SC lattices displaced by ( ½ , ½, ½) 2 ions per cubic cell Cs+ + - 01 Cs ions Cl ions (0, 0, 0) ½ (½, ½, ½) 01 Cl- 01 01
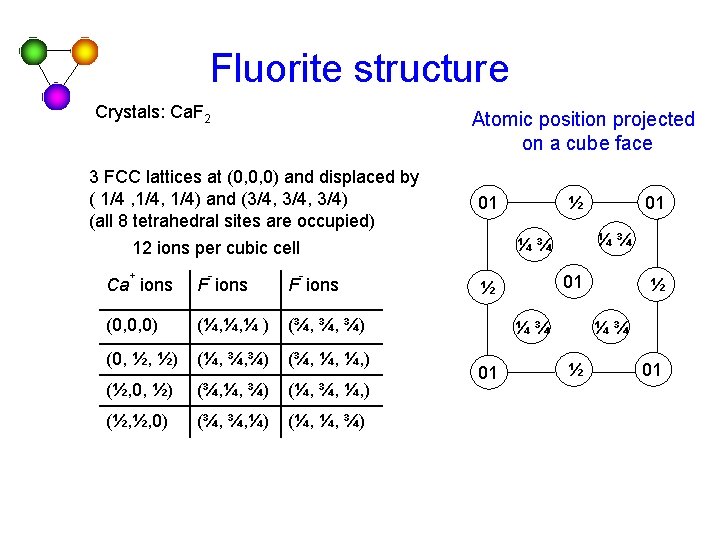
Fluorite structure Crystals: Ca. F 2 Atomic position projected on a cube face 3 FCC lattices at (0, 0, 0) and displaced by ( 1/4 , 1/4) and (3/4, 3/4) (all 8 tetrahedral sites are occupied) 01 + - - F ions (0, 0, 0) (¼, ¼, ¼ ) (¾, ¾, ¾) (0, ½, ½) (¼, ¾, ¾) (¾, ¼, ¼, ) (½, 0, ½) (¾, ¼, ¾) (¼, ¾, ¼, ) (½, ½, 0) (¾, ¾, ¼) (¼, ¼, ¾) 01 ½ ¼¾ 01 01 ¼¾ ¼¾ 12 ions per cubic cell Ca ions ½ ½ ¼¾ ½ 01
- Slides: 11