Primer Selection For any process the primer selected








- Slides: 28

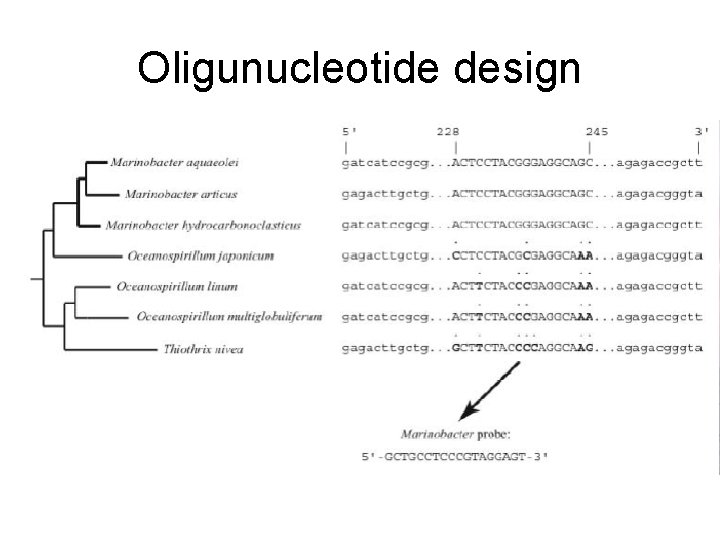
Primer Selection • For any process, the primer selected for use in PCR to amplify some piece of DNA is the first point that determines what sort of information you will be working with • Universal for ID, targeted for group selection, gene group targeting (NOT necessarily expressed though)

Bias • Bias occurs for any reaction – some organisms will be selected over others in a way that changes their apparent abundance • PCR – selects for organisms in higher abundance (not good to ID organisms at 0. x-5%, roughly) some organisms hybridize to primers faster, conditions through PCR reaction can affect…

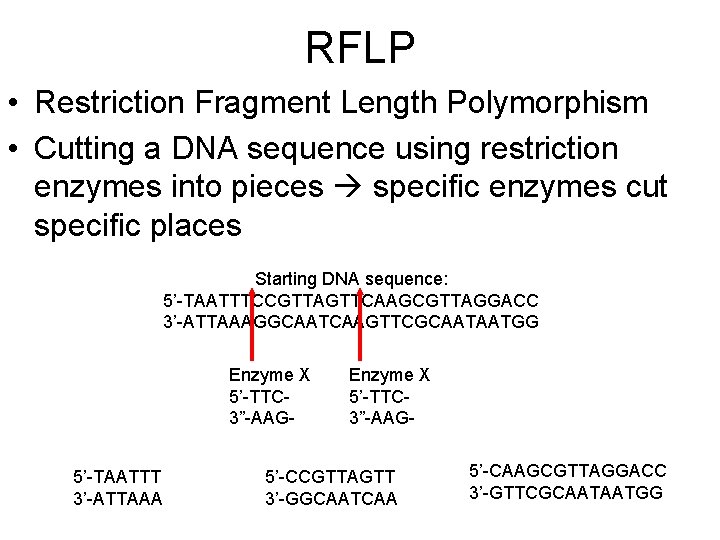
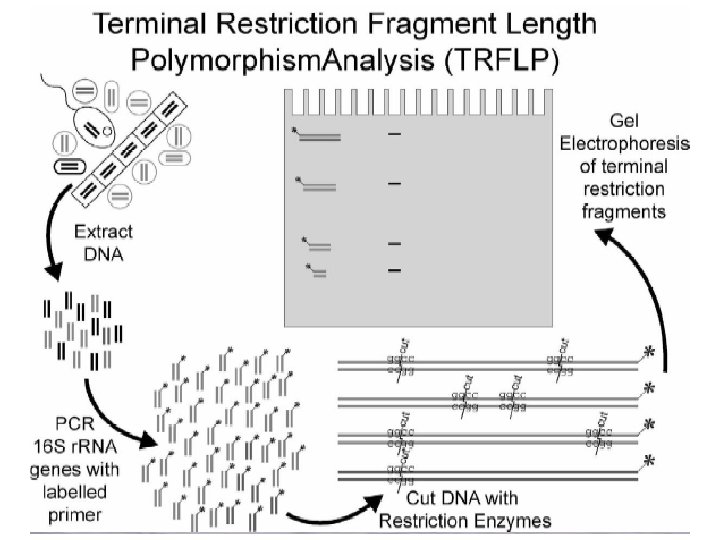
RFLP • Restriction Fragment Length Polymorphism • Cutting a DNA sequence using restriction enzymes into pieces specific enzymes cut specific places Starting DNA sequence: 5’-TAATTTCCGTTAGTTCAAGCGTTAGGACC 3’-ATTAAAGGCAATCAAGTTCGCAATAATGG Enzyme X 5’-TTC 3”-AAG 5’-TAATTT 3’-ATTAAA Enzyme X 5’-TTC 3”-AAG- 5’-CCGTTAGTT 3’-GGCAATCAA 5’-CAAGCGTTAGGACC 3’-GTTCGCAATAATGG

RFLP • DNA can be processed by RFLP either directly (if you can get enough DNA from an environment) or from PCR product • T-RFLP (terminal-RFLP) is in most respects identical except for a marker on the end of the enzyme • Works as fingerprinting technique because different organisms with different DNA sequences will have different lengths of DNA between identical units targeted by the restriction enzymes – specificity can again be manipulated with PCR primers Liu et al. (1997) Appl Environ Microbiol 63: 4516 -4522

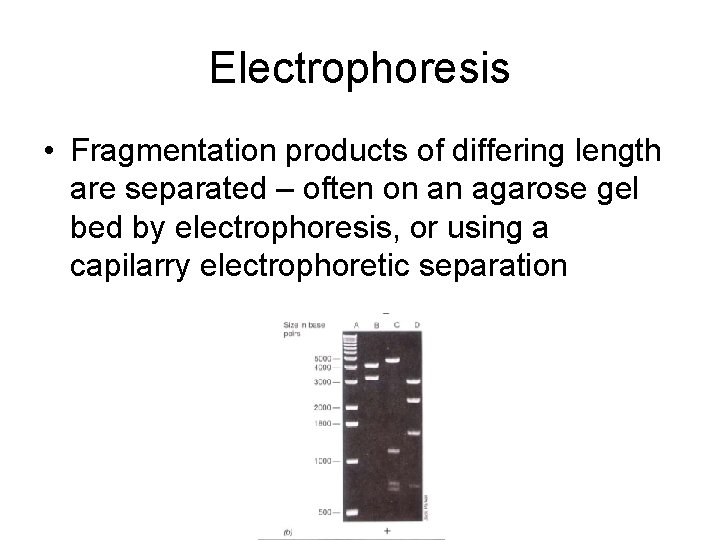
Electrophoresis • Fragmentation products of differing length are separated – often on an agarose gel bed by electrophoresis, or using a capilarry electrophoretic separation


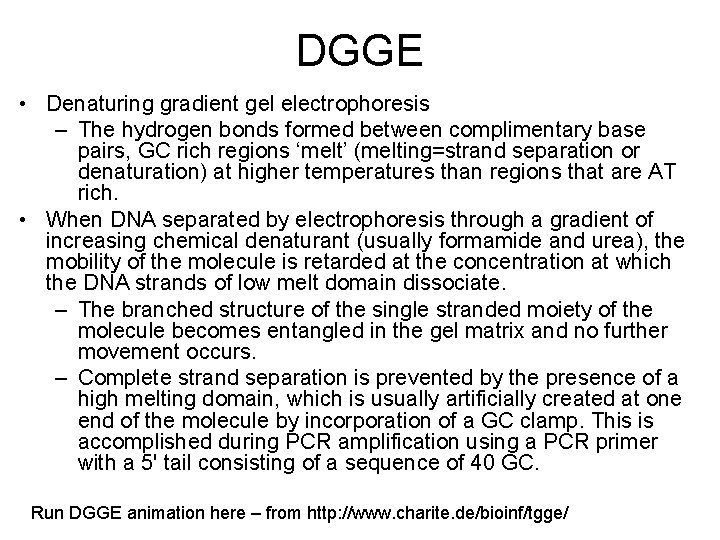
DGGE • Denaturing gradient gel electrophoresis – The hydrogen bonds formed between complimentary base pairs, GC rich regions ‘melt’ (melting=strand separation or denaturation) at higher temperatures than regions that are AT rich. • When DNA separated by electrophoresis through a gradient of increasing chemical denaturant (usually formamide and urea), the mobility of the molecule is retarded at the concentration at which the DNA strands of low melt domain dissociate. – The branched structure of the single stranded moiety of the molecule becomes entangled in the gel matrix and no further movement occurs. – Complete strand separation is prevented by the presence of a high melting domain, which is usually artificially created at one end of the molecule by incorporation of a GC clamp. This is accomplished during PCR amplification using a PCR primer with a 5' tail consisting of a sequence of 40 GC. Run DGGE animation here – from http: //www. charite. de/bioinf/tgge/

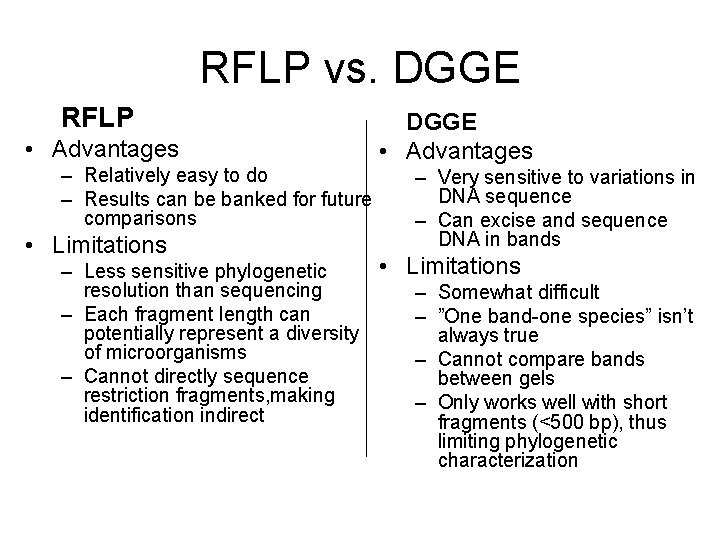
RFLP vs. DGGE RFLP • Advantages – Relatively easy to do – Results can be banked for future comparisons • Limitations DGGE • Advantages – Very sensitive to variations in DNA sequence – Can excise and sequence DNA in bands • Limitations – Less sensitive phylogenetic resolution than sequencing – Somewhat difficult – Each fragment length can – ”One band-one species” isn’t potentially represent a diversity always true of microorganisms – Cannot compare bands – Cannot directly sequence between gels restriction fragments, making – Only works well with short identification indirect fragments (<500 bp), thus limiting phylogenetic characterization

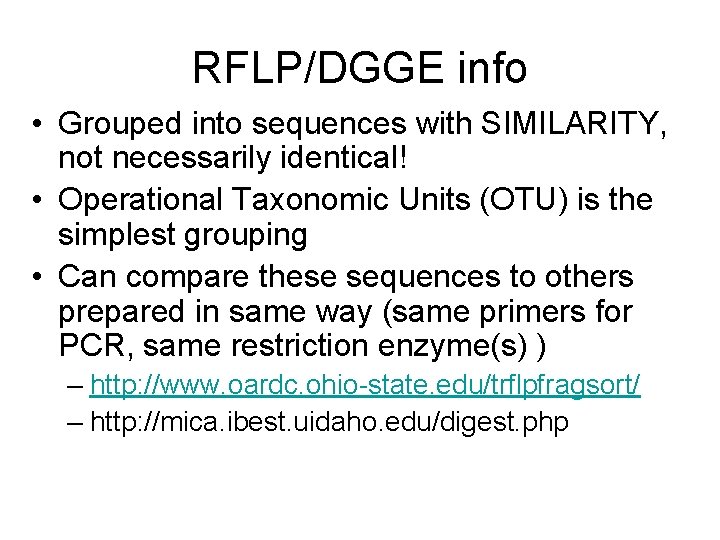
RFLP/DGGE info • Grouped into sequences with SIMILARITY, not necessarily identical! • Operational Taxonomic Units (OTU) is the simplest grouping • Can compare these sequences to others prepared in same way (same primers for PCR, same restriction enzyme(s) ) – http: //www. oardc. ohio-state. edu/trflpfragsort/ – http: //mica. ibest. uidaho. edu/digest. php

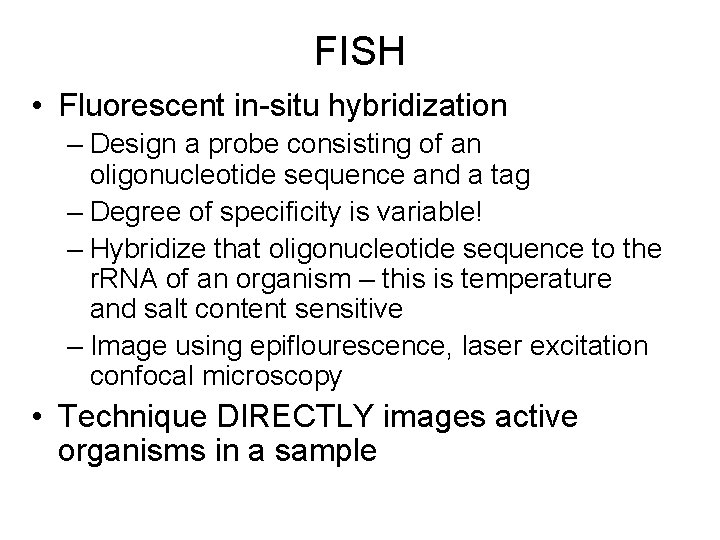
FISH • Fluorescent in-situ hybridization – Design a probe consisting of an oligonucleotide sequence and a tag – Degree of specificity is variable! – Hybridize that oligonucleotide sequence to the r. RNA of an organism – this is temperature and salt content sensitive – Image using epiflourescence, laser excitation confocal microscopy • Technique DIRECTLY images active organisms in a sample

Fluorescent in site hybridization

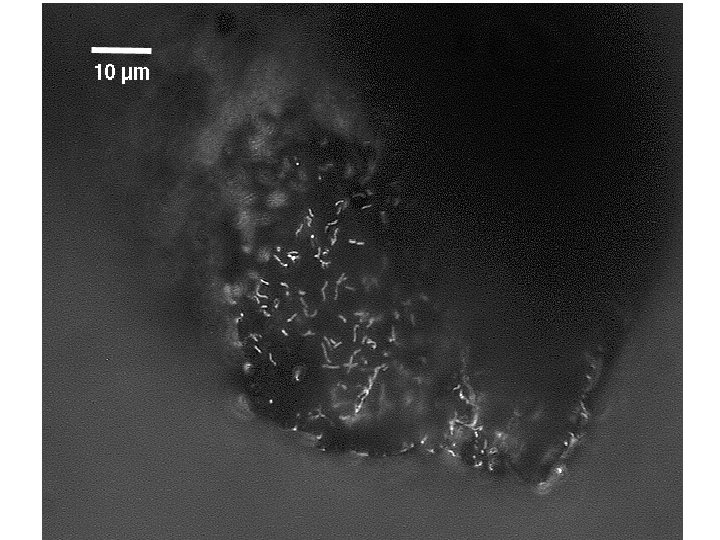
B Drift Slime Streamer 10 µm DAPI FER 656


Oligunucleotide design

FISH variations • FISH-CARD – instead of a fluorescent probe on oligo sequence, but another molecule that can then bond to many fluorescent probes – better signal-to-noise ratio • FISH-RING – design of oligo sequence to specific genes – image all organisms with DSR gene or nif. H for example

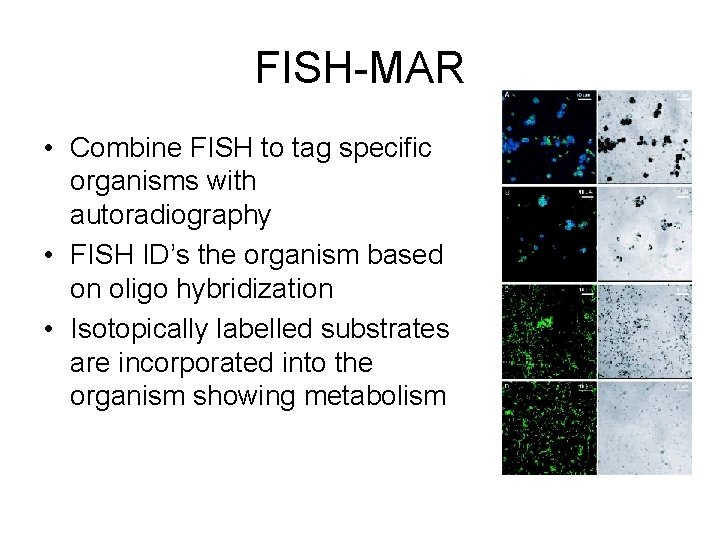
FISH-MAR • Combine FISH to tag specific organisms with autoradiography • FISH ID’s the organism based on oligo hybridization • Isotopically labelled substrates are incorporated into the organism showing metabolism

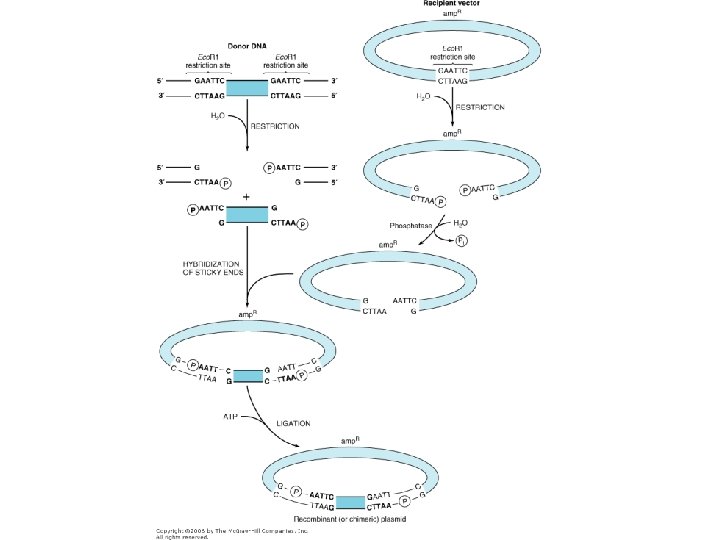
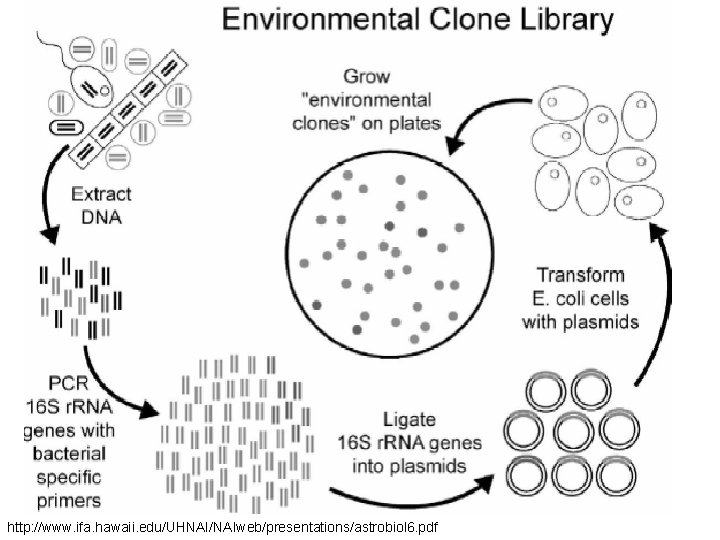
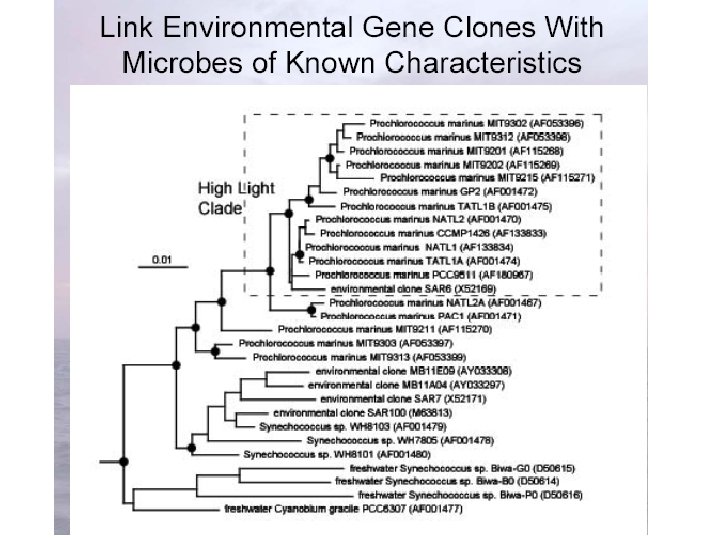
Phylogenetic Data • Numerous techniques to get at the sequences: – PCR – Bacterial Artificial Chromosome (BAC) libraries – Shotgun sequencing


http: //www. ifa. hawaii. edu/UHNAI/NAIweb/presentations/astrobiol 6. pdf

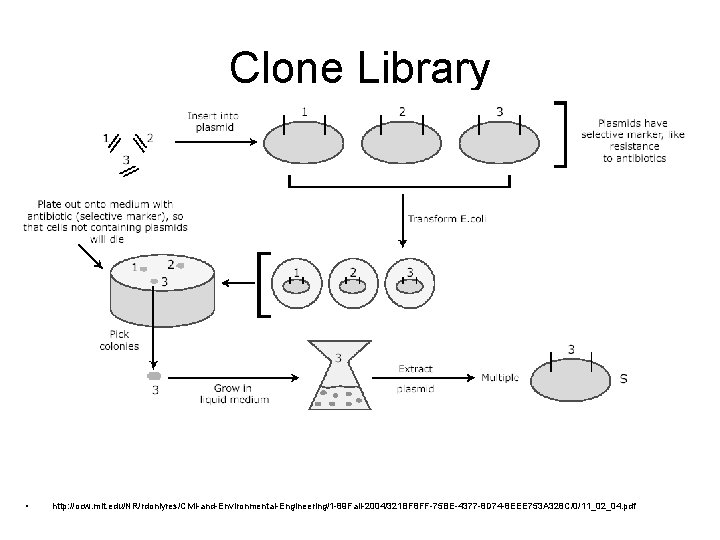
Clone Library • http: //ocw. mit. edu/NR/rdonlyres/Civil-and-Environmental-Engineering/1 -89 Fall-2004/321 BF 8 FF-75 BE-4377 -8 D 74 -8 EEE 753 A 328 C/0/11_02_04. pdf

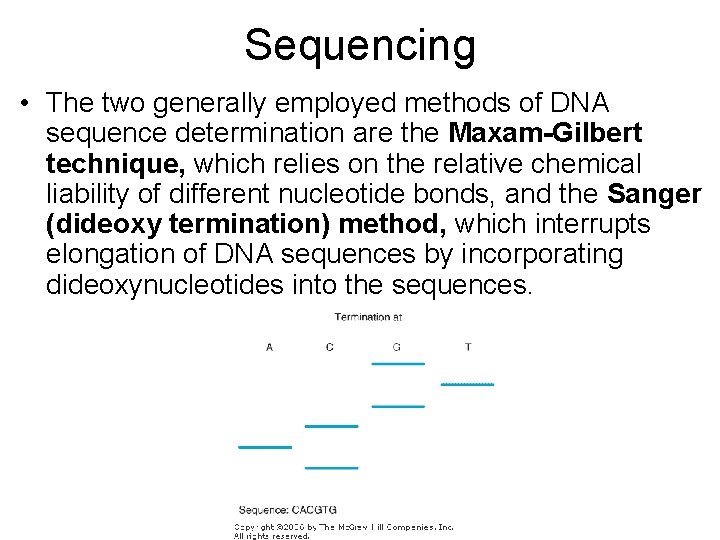
Sequencing • The two generally employed methods of DNA sequence determination are the Maxam-Gilbert technique, which relies on the relative chemical liability of different nucleotide bonds, and the Sanger (dideoxy termination) method, which interrupts elongation of DNA sequences by incorporating dideoxynucleotides into the sequences.

Shotgunning • • Take the whole genome Break it into random fragments Sequence those fragments Re-assemble using computers to re-align portions of each fragment to get back to the entire sequence


Culturing techniques • Microbiology owes it’s roots and much information we know about some organisms from culturing techniques • Culturing generally attempts to develop an isolate (not always of course) • Choose medium, environment (chemical and physical) – innoculate and grow… • Plating or streak culturing – smear a sample so it becomes diluted…

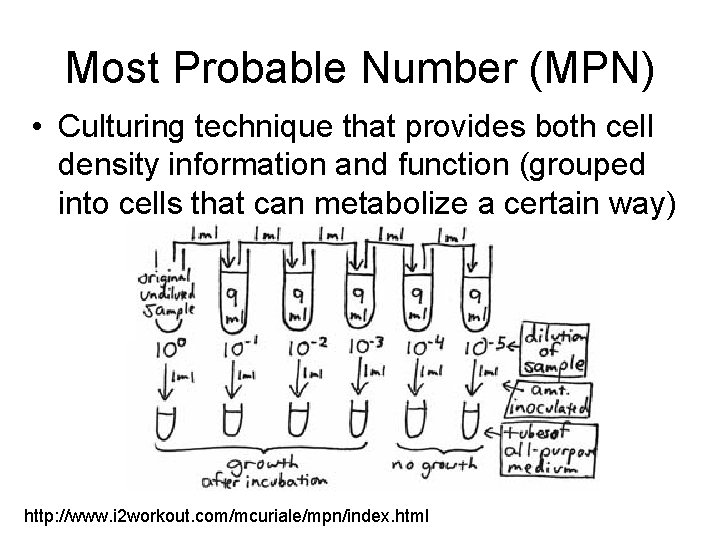
Most Probable Number (MPN) • Culturing technique that provides both cell density information and function (grouped into cells that can metabolize a certain way) http: //www. i 2 workout. com/mcuriale/mpn/index. html

Environmental Sampling • As soon as you remove organisms from their surroundings, they DO NOT just stop acting – often changing function and ecology quickly • ‘Fixing’ is the technique of preserving the cells as close to their actual distribution as possible – Physical (freezing) – Chemical (additives to arrest function) – These techniques kill all, or at least most, cells • Preservation – most also preserve the material you are after until you get to the lab

Materials • Fixation: – Freezing – How fast? Dry ice-ethanol, liquid nitrogen – Chemical – Ethanol, Glutaraldehyde, Parafomaldehyde • Preservation – – Freezing Ethanol RNAlater Salts, buffers

Sterile technique • When should we be MOST concerned with maintaining sterile technique? – SEM samples – PCR samples – FISH samples – MPN samples – Culture samples – Shotgun samples