Primary Endovascular Treatment of FemoroPopliteal TASC C D





![12 -month Primary Patency [180 / 220 pts] Preliminary 180 patients Zilver PTX : 12 -month Primary Patency [180 / 220 pts] Preliminary 180 patients Zilver PTX :](https://slidetodoc.com/presentation_image_h/da95edf84d659ca6fe5633b6edbdd150/image-24.jpg)
![12 -month freedom from TLR [180 / 220 pts] Zilver PTX : 82. 40 12 -month freedom from TLR [180 / 220 pts] Zilver PTX : 82. 40](https://slidetodoc.com/presentation_image_h/da95edf84d659ca6fe5633b6edbdd150/image-25.jpg)
![24 -month Primary Patency [110 / 220 pts] Preliminary 110 patients Zilver PTX : 24 -month Primary Patency [110 / 220 pts] Preliminary 110 patients Zilver PTX :](https://slidetodoc.com/presentation_image_h/da95edf84d659ca6fe5633b6edbdd150/image-27.jpg)
![24 -month freedom from TLR [110 / 220 pts] Zilver PTX : 80. 40 24 -month freedom from TLR [110 / 220 pts] Zilver PTX : 80. 40](https://slidetodoc.com/presentation_image_h/da95edf84d659ca6fe5633b6edbdd150/image-28.jpg)
- Slides: 29

Primary Endovascular Treatment of Femoro-Popliteal TASC C & D Lesions Similar Effective as Bypass Surgery Prof. Thomas Zeller, MD, Ph. D Department Angiology Clinic for Cardiology and Angiology II University Heart-Center Freiburg - Bad Krozingen , Germany

Thomas Zeller, MD For the 12 months preceding this presentation, I disclose the following types of financial relationships: • • Honoraria received from: Abbott Vascular, Bard Peripheral Vascular, Veryan, Biotronik, Boston Scientific Corp. , Cook Medical, Gore & Associates, Medtronic, Philips-Spectranetics, Tri. Reme, Veryan, Shockwave, Biotronik Consulted for: Boston Scientific Corp. , Cook Medical, Gore & Associates, Medtronic, Spectranetics, Veryan Research, clinical trial, or drug study funds received from: Biotronik, 480 biomedical, Bard Peripheral Vascular, Veryan, Biotronik, Cook Medical, Gore & Associates, Abbott Vascular, Medtronic, Philips, Terumo, Tri. Reme, Veryan, Shockwave Common stock: Veryan, QT Medical

TASC C & D SFA Lesions Bypass is considered the golden standard First Author/Yr Type of Study Hines/2010 Retrospective Mc. Quade/2009 Prospective, randomized 86 patients/100 limbs e. PTFE/nitinol stent graft vs AK 50 limbs: stent graft popliteal bypass with synthetic 50 limbs: bypass with Dacron graft or e. PTFE material Prosepctive, Randomized Kedora/2007 Study Population 27 patients with TASC D lesions above- and below-knee venous FP bypass M 2 1 @ P P s as Byp Viabahn stent grafts vs prosthetic femoro-(aboveknee) popliteal bypass Prospective, Randomized: Jensen/2007 Berglund/2005 86 patients with femoro-popliteal artery occlusive disease 50 limbs treated with angio and stent 50 limbs treated with bypass with synthetic Dacron or PTFE grafts % 8 7 / =+ 427 patients with chronic lower limb ischemia with bypass for PTFE and Dacron for above the suprageniculate bypass: 205 with PTFE and 208 with Dacron knee femoropopliteal bypass Retrospective comparison: autologous saphenous vein grafts to PTFE grafts 499 patients undergoing bypassfor CLI or claudication: -139 with vein graft -360 with e. PTFE Bosiers M. LINC 2018 Primary patency 12 months: 73. 2% 12 -month: Stent-graft: 72% Bypass: 77% 12 -month: Stent: 73. 5% Bypass: 74. 2% 12 -month: Dacron: ~78% PTFE: ~70% Vein, claudication: ~87% e. PTFE, claudication: ~75% 3

Analysis of PSV in 100 surgical, primary patent bypasses Total (N=100) Binary restenosis (N=11) F-P 1 37 3 F-P 2 0 0 P P s s a p P y s B s a p y B M 2 F-tibial 16 2 1 @ M @12 % 0 7 / + % 8 = = 89% 100%=surgical +/-7 primary patent endo primary patent F-P 3 P 47 Bosiers M. LINC 20188 6 4

Improved Durability of Endovascular Therapy in TASC C & D Lesions


4. 4. 11

4. 4. 11

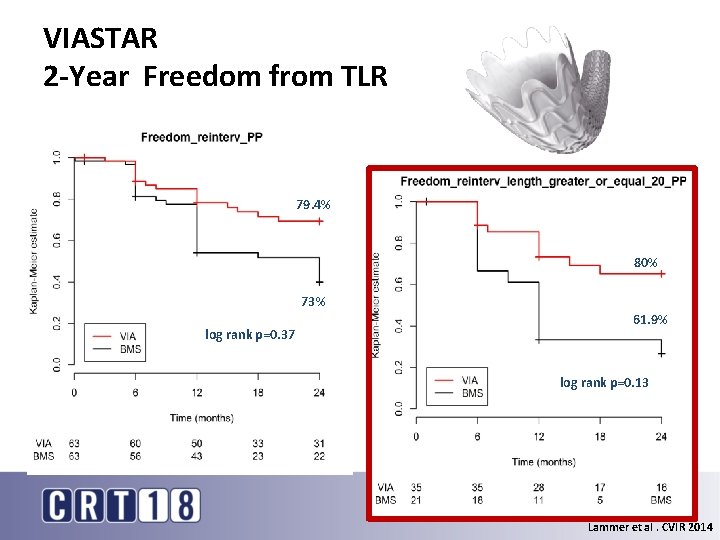
VIASTAR 2 -Year Freedom from TLR 79. 4% 80% 73% log rank p=0. 37 61. 9% log rank p=0. 13 Lammer et al. CVIR 2014

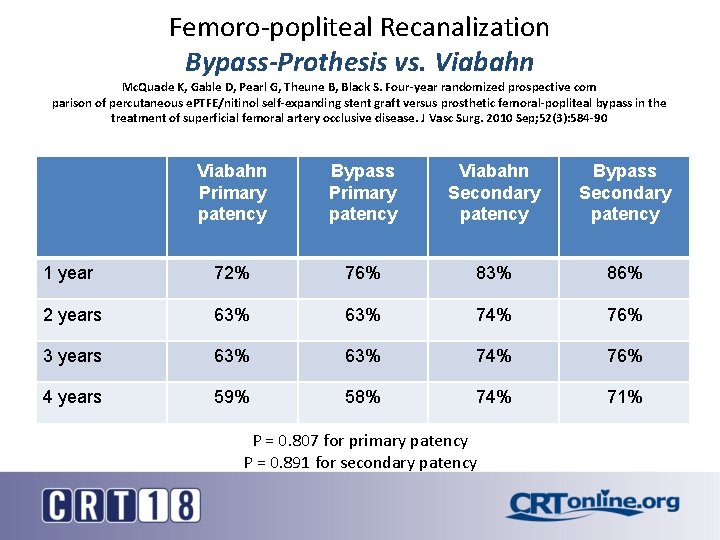
Femoro-popliteal Recanalization Bypass-Prothesis vs. Viabahn Mc. Quade K, Gable D, Pearl G, Theune B, Black S. Four-year randomized prospective com parison of percutaneous e. PTFE/nitinol self-expanding stent graft versus prosthetic femoral-popliteal bypass in the treatment of superficial femoral artery occlusive disease. J Vasc Surg. 2010 Sep; 52(3): 584 -90 Viabahn Primary patency Bypass Primary patency Viabahn Secondary patency Bypass Secondary patency 1 year 72% 76% 83% 86% 2 years 63% 74% 76% 3 years 63% 74% 76% 4 years 59% 58% 74% 71% P = 0. 807 for primary patency P = 0. 891 for secondary patency

Super. B – Viabahn vs. Venous Bypass Graft Study Design Reijnen M. submitted

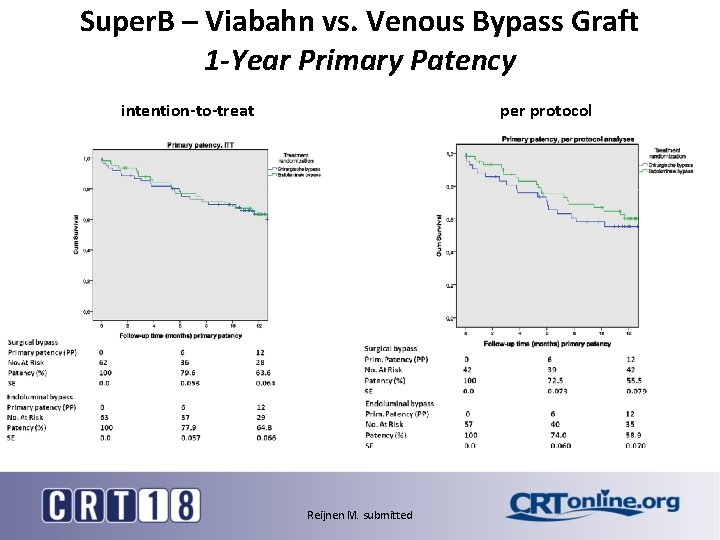
Super. B – Viabahn vs. Venous Bypass Graft 1 -Year Primary Patency intention-to-treat per protocol Reijnen M. submitted

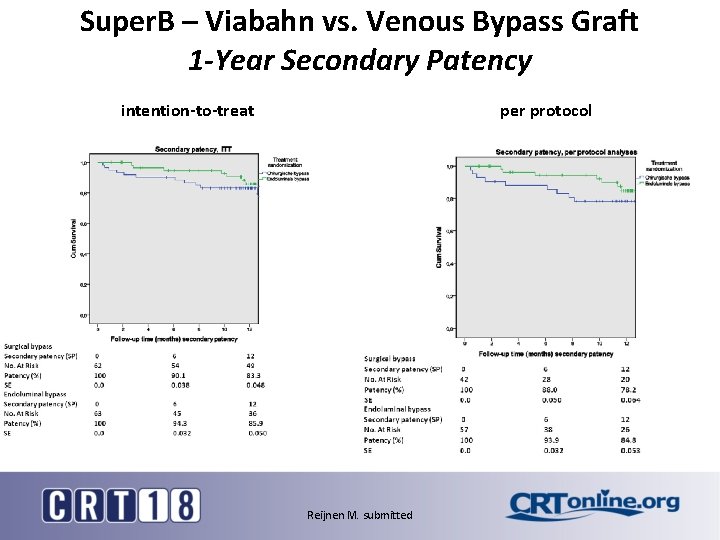
Super. B – Viabahn vs. Venous Bypass Graft 1 -Year Secondary Patency intention-to-treat per protocol Reijnen M. submitted

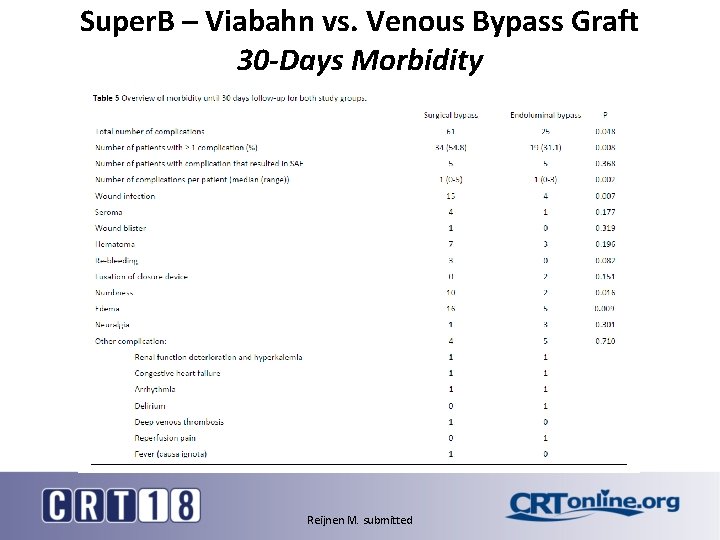
Super. B – Viabahn vs. Venous Bypass Graft 30 -Days Morbidity Reijnen M. submitted

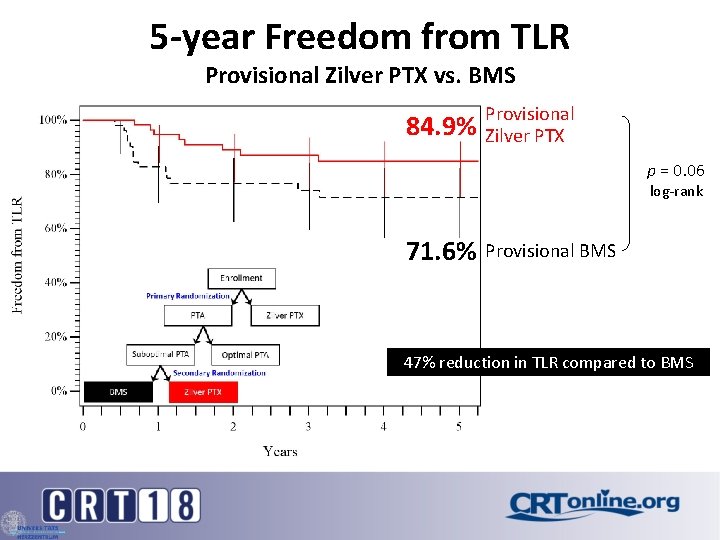
5 -year Freedom from TLR Provisional Zilver PTX vs. BMS 84. 9% Provisional Zilver PTX p = 0. 06 log-rank 71. 6% Provisional BMS 47% reduction in TLR compared to BMS

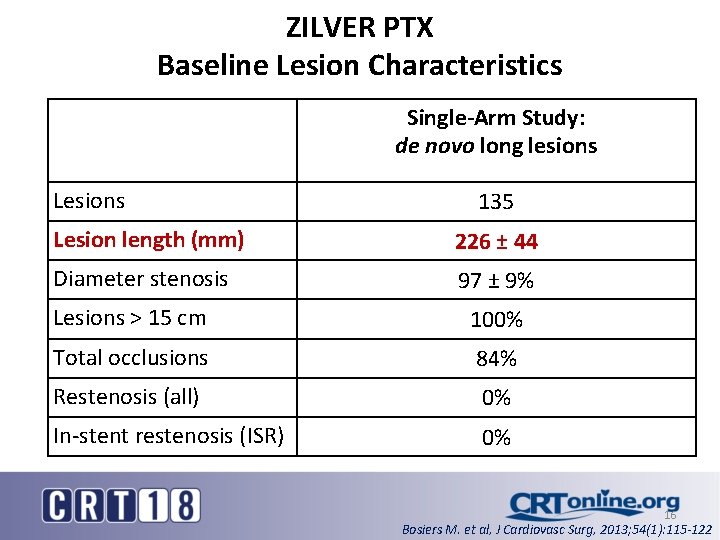
ZILVER PTX Baseline Lesion Characteristics Single-Arm Study: de novo long lesions Lesions 135 Lesion length (mm) 226 ± 44 Diameter stenosis 97 ± 9% Lesions > 15 cm 100% Total occlusions 84% Restenosis (all) 0% In-stent restenosis (ISR) 0% 16 Bosiers M. et al, J Cardiovasc Surg, 2013; 54(1): 115 -122

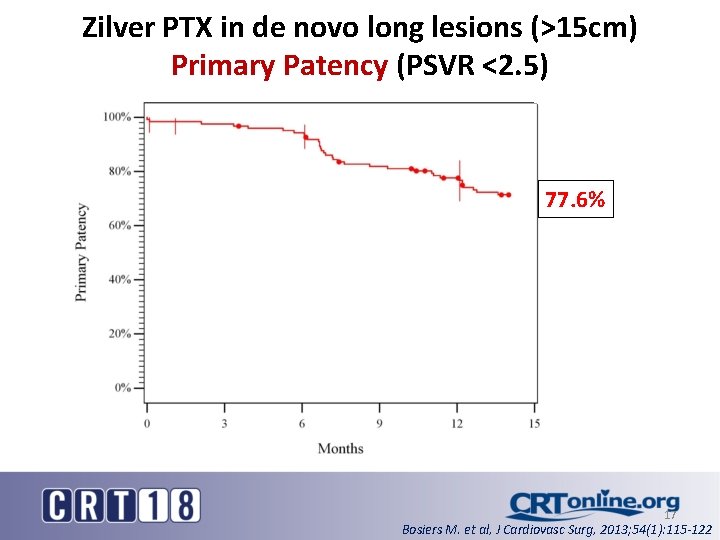
Zilver PTX in de novo long lesions (>15 cm) Primary Patency (PSVR <2. 5) 77. 6% 17 Bosiers M. et al, J Cardiovasc Surg, 2013; 54(1): 115 -122

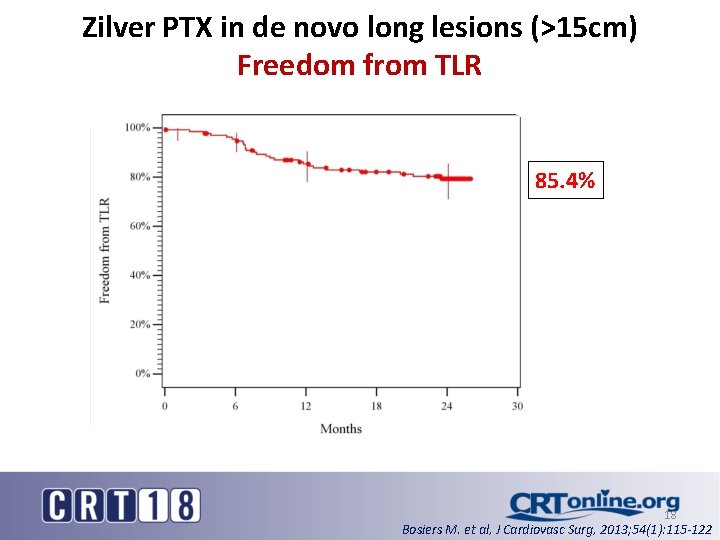
Zilver PTX in de novo long lesions (>15 cm) Freedom from TLR 85. 4% 18 Bosiers M. et al, J Cardiovasc Surg, 2013; 54(1): 115 -122

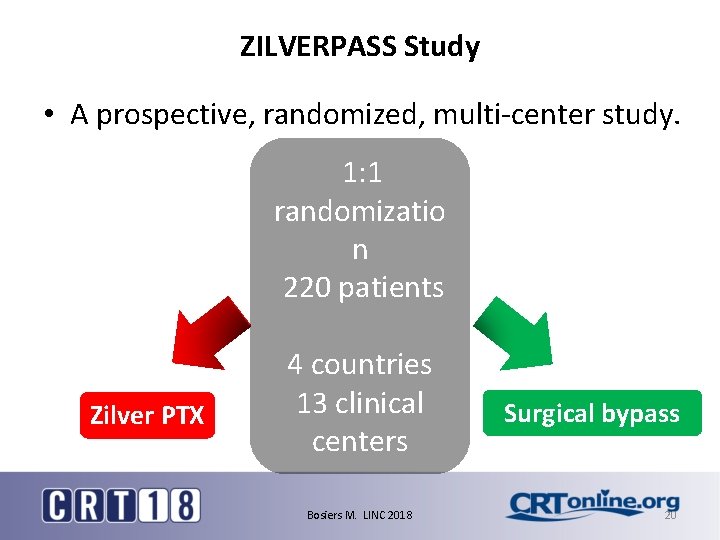
ZILVERPASS study The Cook Zilver PTX drugeluting stent versus bypass surgery for the treatment of femoropopliteal TASC C&D lesions. 19

ZILVERPASS Study • A prospective, randomized, multi-center study. 1: 1 randomizatio n 220 patients Zilver PTX 4 countries 13 clinical centers Bosiers M. LINC 2018 Surgical bypass 20

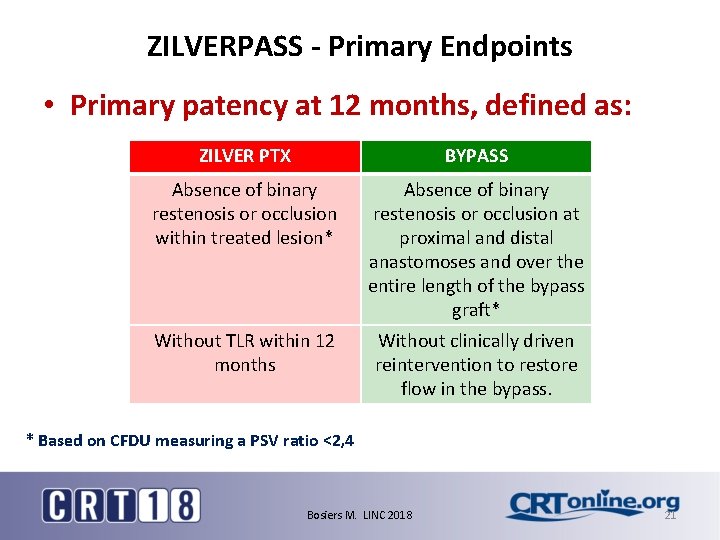
ZILVERPASS - Primary Endpoints • Primary patency at 12 months, defined as: ZILVER PTX BYPASS Absence of binary restenosis or occlusion within treated lesion* Absence of binary restenosis or occlusion at proximal and distal anastomoses and over the entire length of the bypass graft* Without TLR within 12 months Without clinically driven reintervention to restore flow in the bypass. * Based on CFDU measuring a PSV ratio <2, 4 Bosiers M. LINC 2018 21

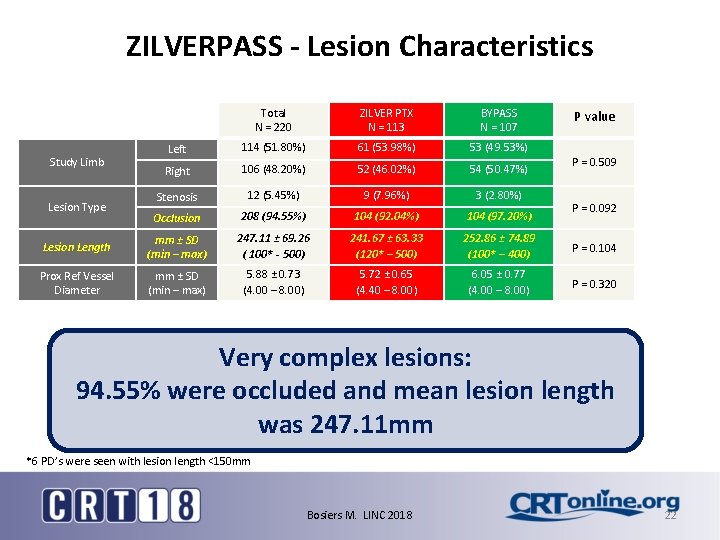
ZILVERPASS - Lesion Characteristics Total N = 220 ZILVER PTX N = 113 BYPASS N = 107 Left 114 (51. 80%) 61 (53. 98%) 53 (49. 53%) Right 106 (48. 20%) 52 (46. 02%) 54 (50. 47%) Stenosis 12 (5. 45%) 9 (7. 96%) 3 (2. 80%) Occlusion 208 (94. 55%) 104 (92. 04%) 104 (97. 20%) Lesion Length mm ± SD (min – max) 247. 11 ± 69. 26 ( 100* - 500) 241. 67 ± 63. 33 (120* – 500) 252. 86 ± 74. 89 (100* – 400) P = 0. 104 Prox Ref Vessel Diameter mm ± SD (min – max) 5. 88 ± 0. 73 (4. 00 – 8. 00) 5. 72 ± 0. 65 (4. 40 – 8. 00) 6. 05 ± 0. 77 (4. 00 – 8. 00) P = 0. 320 Study Limb Lesion Type P value P = 0. 509 P = 0. 092 Very complex lesions: 94. 55% were occluded and mean lesion length was 247. 11 mm *6 PD’s were seen with lesion length <150 mm Bosiers M. LINC 2018 22

ZILVERPASS - Procedure Characteristics Procedure Time Bypass material Hospital stay* minutes ± SD (min – max) Total N = 220 ZILVER PTX N = 113 BYPASS N = 107 90. 46 ± 44. 77 (17 – 240) 59. 60 ± 22. 65 (17 – 135) 123. 05 ± 38. 88 (53 – 240) Dacron 42 (39. 25%) PTFE 65 (60. 75%) Nights (min – max) 5. 26 ± 5. 65 (0. 00 – 34. 00) 2. 52 ± 3. 50 (0. 00 – 20. 00) 8. 14 ± 6. 03 (1. 00 – 34. 00) P value P < 0. 001 P<0. 001 *Data on hospital stay for 219 patients. 1 Patient (ZILVER PTX group) died during hospital stay Bosiers M. LINC 2018 23
![12 month Primary Patency 180 220 pts Preliminary 180 patients Zilver PTX 12 -month Primary Patency [180 / 220 pts] Preliminary 180 patients Zilver PTX :](https://slidetodoc.com/presentation_image_h/da95edf84d659ca6fe5633b6edbdd150/image-24.jpg)
12 -month Primary Patency [180 / 220 pts] Preliminary 180 patients Zilver PTX : 78. 10 % Primary Patency, defined as absence of binary restenosis in both groups ZILVER PTX BYPASS : 73. 10% Baseline 30 days 6 MFU 12 MFU – D 365 12 MFU – D 395 Tar 91 89 83 66 63 % 100 94. 40 78. 10 74. 50 Tar 89 86 75 62 59 % 100 73. 10 70. 60 97. 70 FMRP - 2018 87. 30 24 Bosiers M. LINC 2018
![12 month freedom from TLR 180 220 pts Zilver PTX 82 40 12 -month freedom from TLR [180 / 220 pts] Zilver PTX : 82. 40](https://slidetodoc.com/presentation_image_h/da95edf84d659ca6fe5633b6edbdd150/image-25.jpg)
12 -month freedom from TLR [180 / 220 pts] Zilver PTX : 82. 40 % Preliminary 180 patients BYPASS : 76. 30% ZILVER PTX BYPASS Baseline 30 days 6 MFU 12 MFU – D 365 12 MFU – D 395 Tar 91 89 84 67 66 % 100 95. 00 82. 40 81. 10 Tar 89 87 75 64 62 % 100 98. 90 88. 30 76. 30 FMRP - 2018 25 Bosiers M. LINC 2018

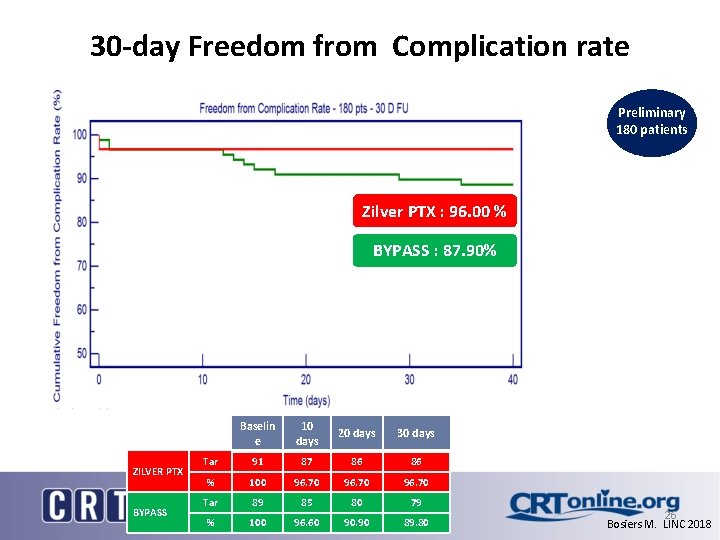
30 -day Freedom from Complication rate Preliminary 180 patients Zilver PTX : 96. 00 % BYPASS : 87. 90% ZILVER PTX BYPASS Baselin e 10 days 20 days 30 days Tar 91 87 86 86 % 100 96. 70 Tar 89 85 80 79 % 100 96. 60 90. 90 89. 80 FMRP - 2018 26 Bosiers M. LINC 2018
![24 month Primary Patency 110 220 pts Preliminary 110 patients Zilver PTX 24 -month Primary Patency [110 / 220 pts] Preliminary 110 patients Zilver PTX :](https://slidetodoc.com/presentation_image_h/da95edf84d659ca6fe5633b6edbdd150/image-27.jpg)
24 -month Primary Patency [110 / 220 pts] Preliminary 110 patients Zilver PTX : 68. 20 % BYPASS : 63. 70% Primary Patency, defined as absence of binary restenosis in both groups ZILVER PTX BYPASS Baseline 30 days 6 MFU 12 MFU 24 MFU Tar 52 51 49 38 28 % 100 98. 00 77. 60 68. 20 Tar 58 56 46 40 30 % 100 98. 20 82. 30 71. 40 63. 70 FMRP - 2018 27 Bosiers M. LINC 2018
![24 month freedom from TLR 110 220 pts Zilver PTX 80 40 24 -month freedom from TLR [110 / 220 pts] Zilver PTX : 80. 40](https://slidetodoc.com/presentation_image_h/da95edf84d659ca6fe5633b6edbdd150/image-28.jpg)
24 -month freedom from TLR [110 / 220 pts] Zilver PTX : 80. 40 % Preliminary 110 patients BYPASS : 70. 30% ZILVER PTX BYPASS Baseline 30 days 6 MFU 12 MFU 24 MFU Tar 52 51 50 40 31 % 100 100 85. 40 80. 40 Tar 58 56 46 42 32 % 100 98. 20 83. 80 76. 30 70. 30 FMRP - 2018 28 Bosiers M. LINC 2018

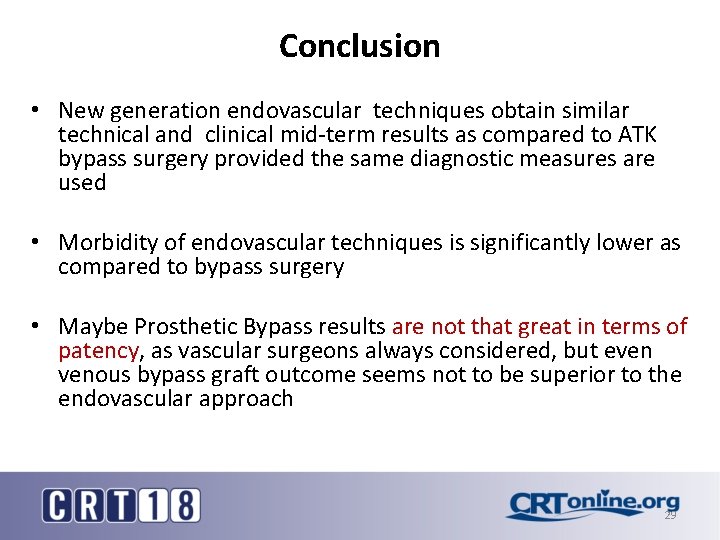
Conclusion • New generation endovascular techniques obtain similar technical and clinical mid-term results as compared to ATK bypass surgery provided the same diagnostic measures are used • Morbidity of endovascular techniques is significantly lower as compared to bypass surgery • Maybe Prosthetic Bypass results are not that great in terms of patency, as vascular surgeons always considered, but even venous bypass graft outcome seems not to be superior to the endovascular approach 29