Prevention of Bleeding in Patients with Atrial Fibrillation
![Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI [PIONEER AF-PCI] Brandon Martinez, Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI [PIONEER AF-PCI] Brandon Martinez,](https://slidetodoc.com/presentation_image_h/84061e6965a0b0a2b3e0daadf03ef019/image-1.jpg)
Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI [PIONEER AF-PCI] Brandon Martinez, Pharm. D, BCPS PGY-2 Cardiology Pharmacy Resident Abbott Northwestern Hospital/Minneapolis Heart Institute Minneapolis, MN Denise Sutter, Pharm. D, BCPS Clinical Pharmacist Specialist, Cardiology/Internal Medicine Detroit Receiving Hospital Detroit, MI

Disclosure • I have nothing to disclose 2
Background Clotting Bleeding coronary • 5 -8% of patients who undergo percutaneous intervention (PCI) have atrial fibrillation Ischemic Stroke Stent Thrombosis N Engl J Med 2016; 375: 2423 -34 Bleeding 3
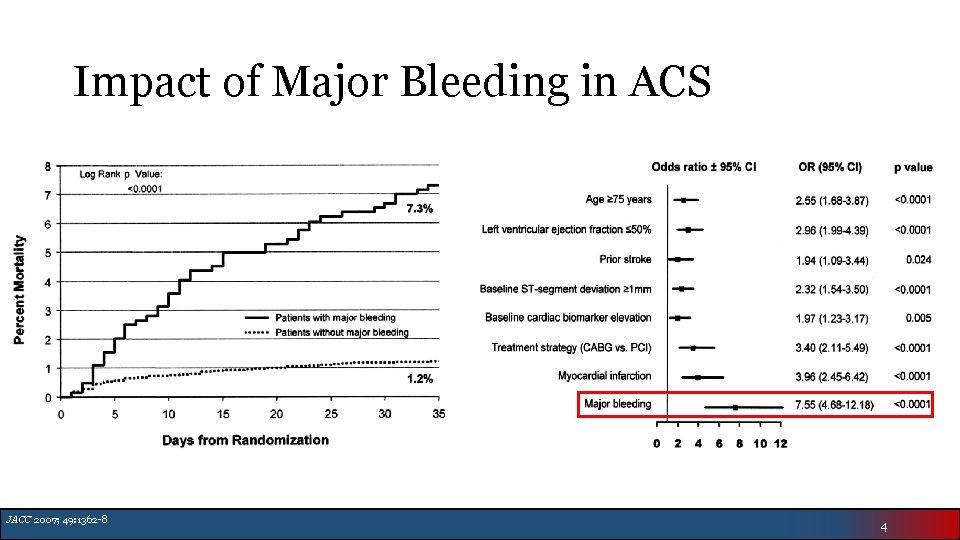
Impact of Major Bleeding in ACS JACC 2007; 49: 1362 -8 4
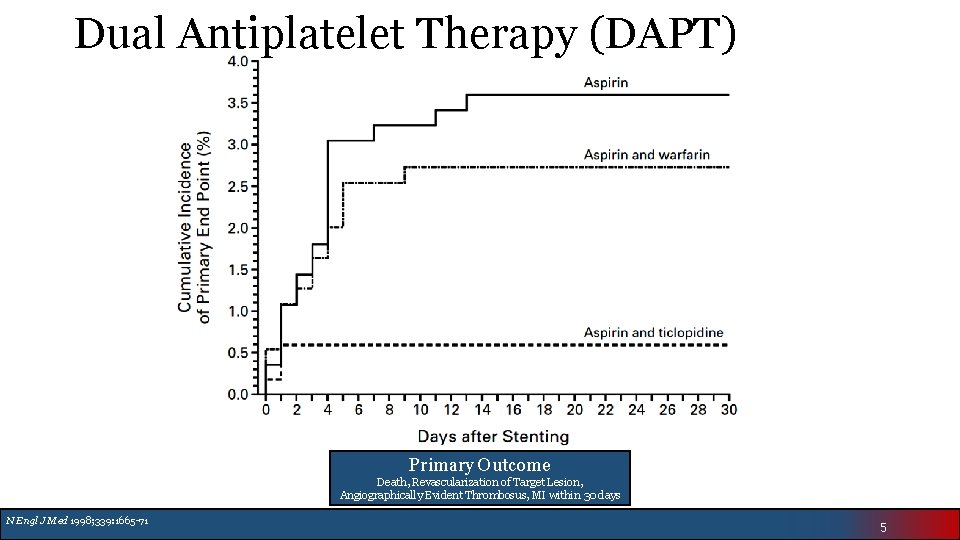
Dual Antiplatelet Therapy (DAPT) Primary Outcome Death, Revascularization of Target Lesion, Angiographically Evident Thrombosus, MI within 30 days N Engl J Med 1998; 339: 1665 -71 5
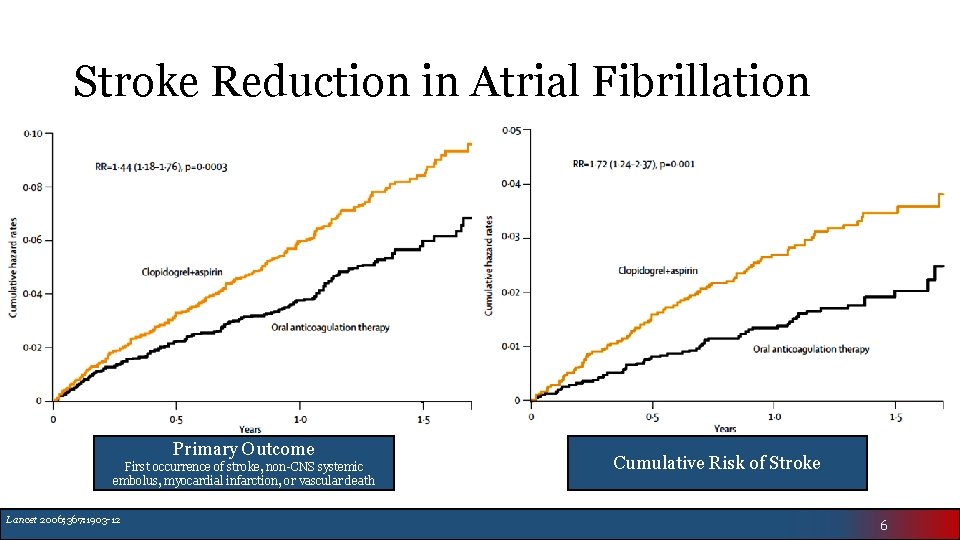
Stroke Reduction in Atrial Fibrillation Primary Outcome First occurrence of stroke, non-CNS systemic embolus, myocardial infarction, or vascular death Lancet 2006; 367: 1903 -12 Cumulative Risk of Stroke 6
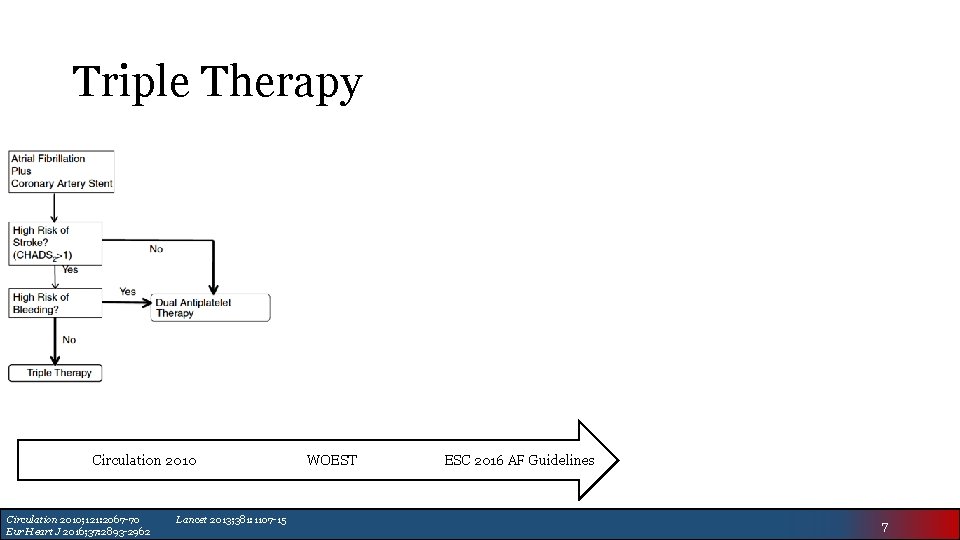
Triple Therapy Circulation 2010; 121: 2067 -70 Eur Heart J 2016; 37: 2893 -2962 Lancet 2013; 381: 1107 -15 WOEST ESC 2016 AF Guidelines 7
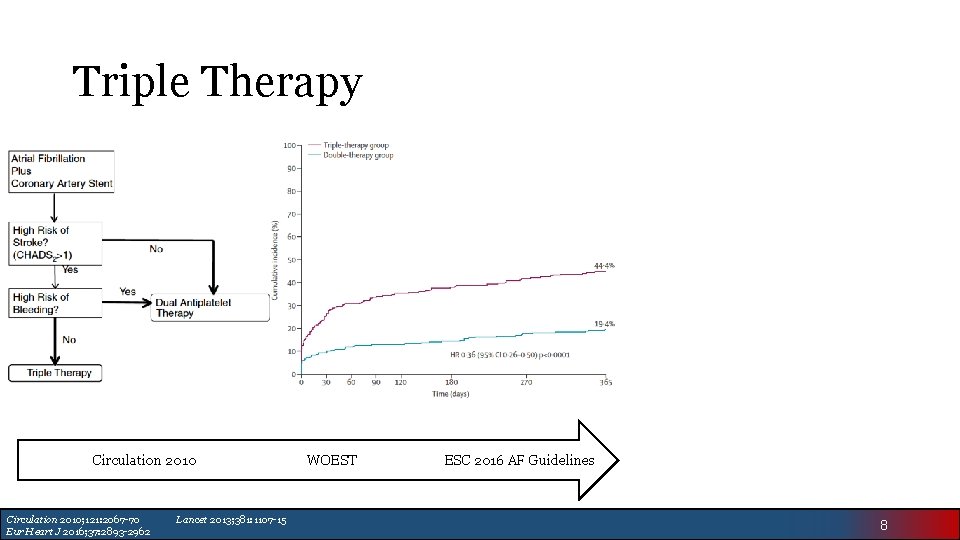
Triple Therapy Circulation 2010; 121: 2067 -70 Eur Heart J 2016; 37: 2893 -2962 Lancet 2013; 381: 1107 -15 WOEST ESC 2016 AF Guidelines 8
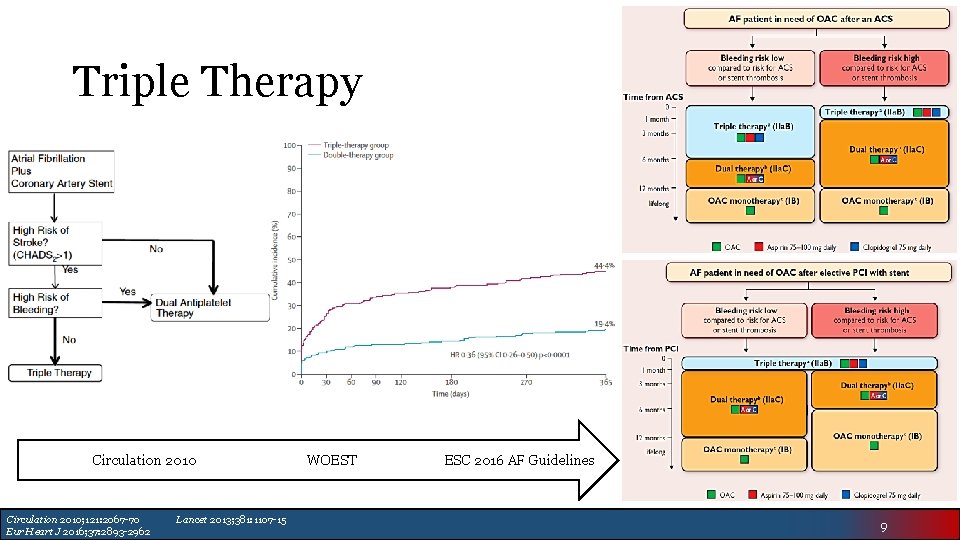
Triple Therapy Circulation 2010; 121: 2067 -70 Eur Heart J 2016; 37: 2893 -2962 Lancet 2013; 381: 1107 -15 WOEST ESC 2016 AF Guidelines 9
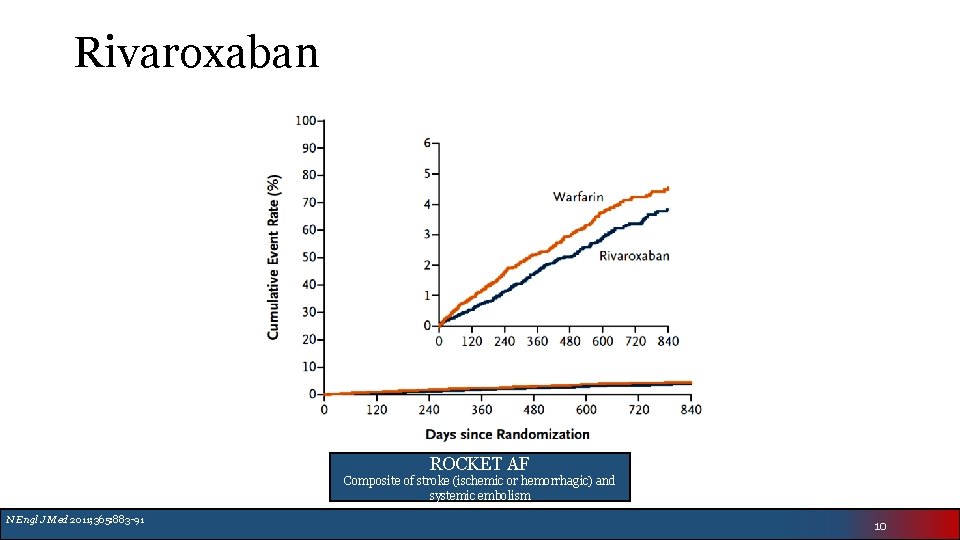
Rivaroxaban ROCKET AF Composite of stroke (ischemic or hemorrhagic) and systemic embolism N Engl J Med 2011; 365: 883 -91 10
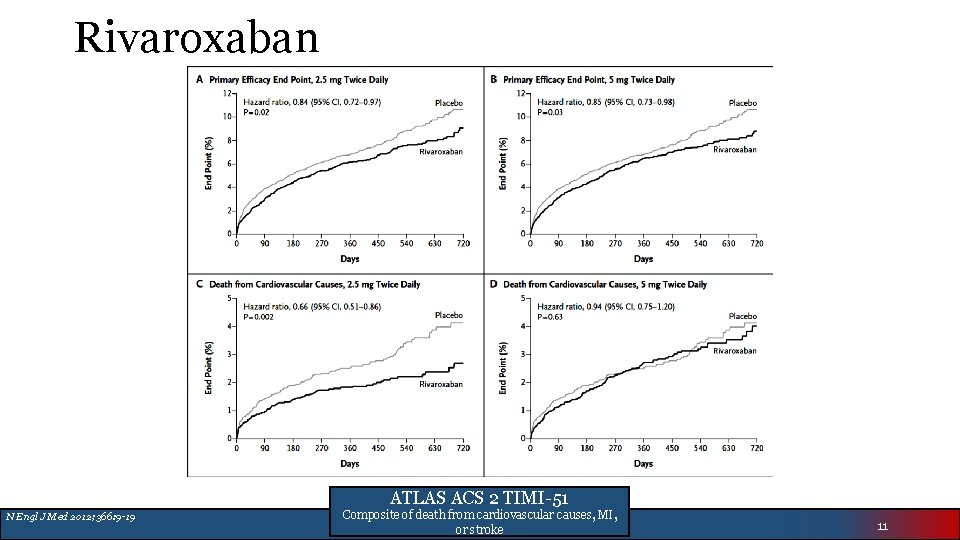
Rivaroxaban ATLAS ACS 2 TIMI-51 N Engl J Med 2012; 366: 9 -19 Composite of death from cardiovascular causes, MI, or stroke 11
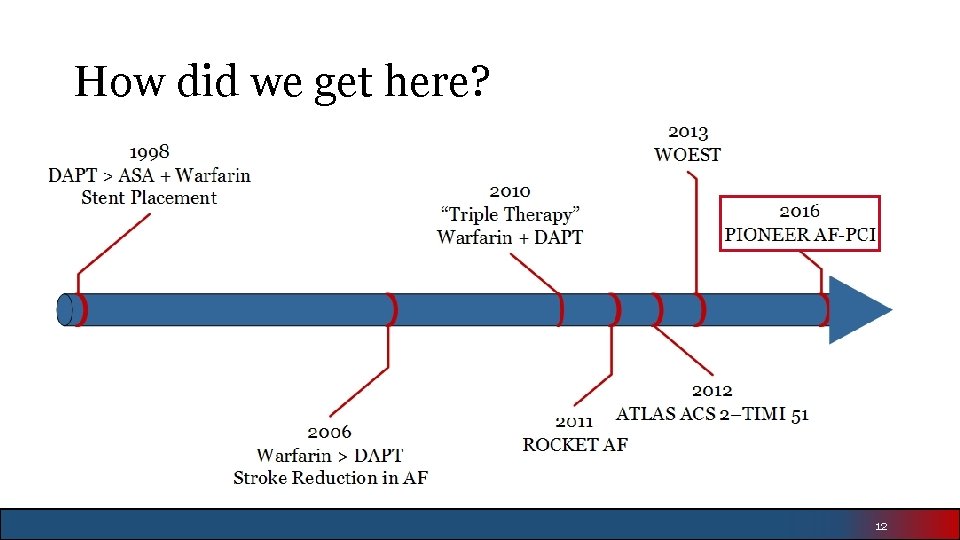
How did we get here? 12
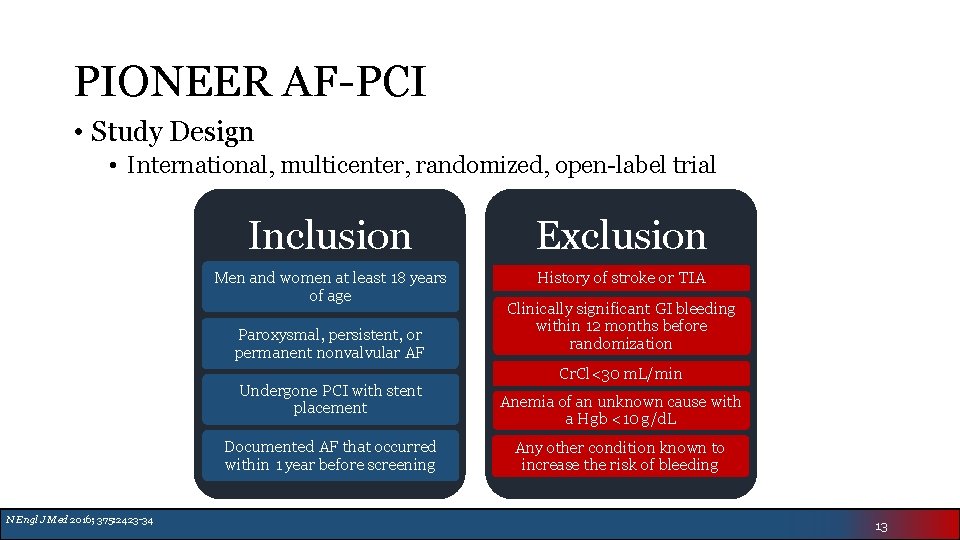
PIONEER AF-PCI • Study Design • International, multicenter, randomized, open-label trial Inclusion Exclusion Men and women at least 18 years of age History of stroke or TIA Paroxysmal, persistent, or permanent nonvalvular AF Undergone PCI with stent placement Documented AF that occurred within 1 year before screening N Engl J Med 2016; 375: 2423 -34 Clinically significant GI bleeding within 12 months before randomization Cr. Cl<30 m. L/min Anemia of an unknown cause with a Hgb <10 g/d. L Any other condition known to increase the risk of bleeding 13
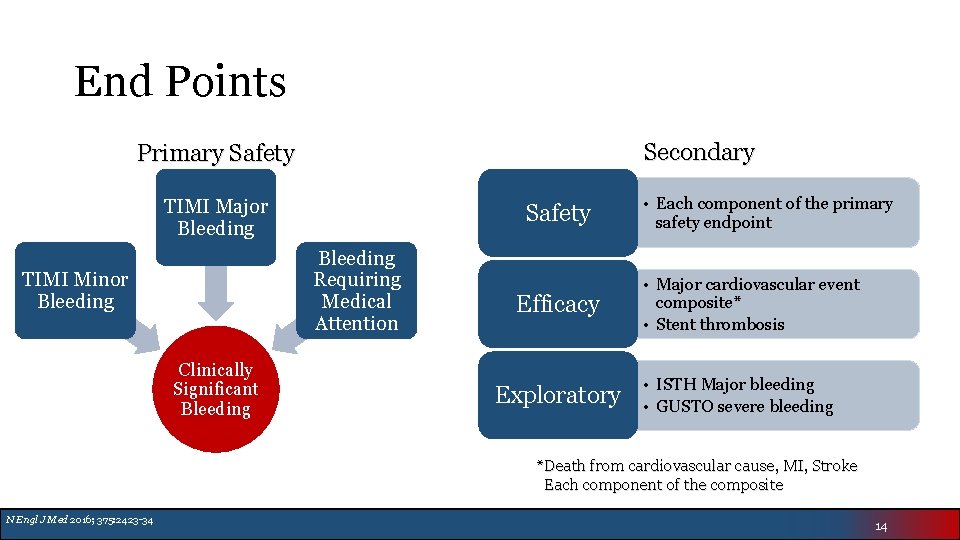
End Points Secondary Primary Safety TIMI Major Bleeding Safety Bleeding Requiring Medical Attention TIMI Minor Bleeding Clinically Significant Bleeding Efficacy Exploratory • Each component of the primary safety endpoint • Major cardiovascular event composite* • Stent thrombosis • ISTH Major bleeding • GUSTO severe bleeding *Death from cardiovascular cause, MI, Stroke Each component of the composite N Engl J Med 2016; 375: 2423 -34 14
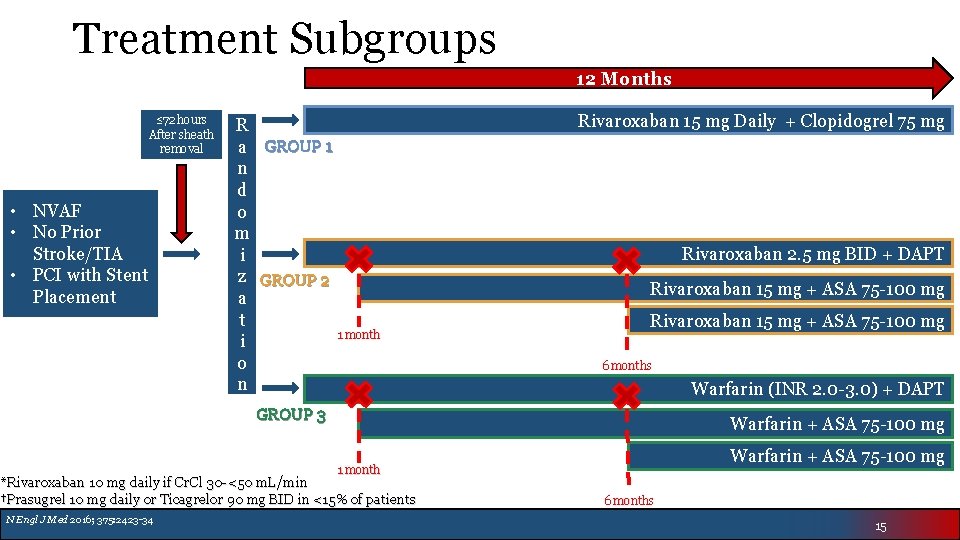
Treatment Subgroups 12 Months ≤ 72 hours After sheath removal • NVAF • No Prior Stroke/TIA • PCI with Stent Placement R a GROUP 1 n d o m i z GROUP 2 a t 1 month i o n Rivaroxaban 15 mg Daily + Clopidogrel 75 mg Rivaroxaban 2. 5 mg BID + DAPT Rivaroxaban 15 mg + ASA 75 -100 mg 6 months Warfarin (INR 2. 0 -3. 0) + DAPT GROUP 3 Warfarin + ASA 75 -100 mg 1 month *Rivaroxaban 10 mg daily if Cr. Cl 30 -<50 m. L/min †Prasugrel 10 mg daily or Ticagrelor 90 mg BID in <15% of patients N Engl J Med 2016; 375: 2423 -34 6 months 15
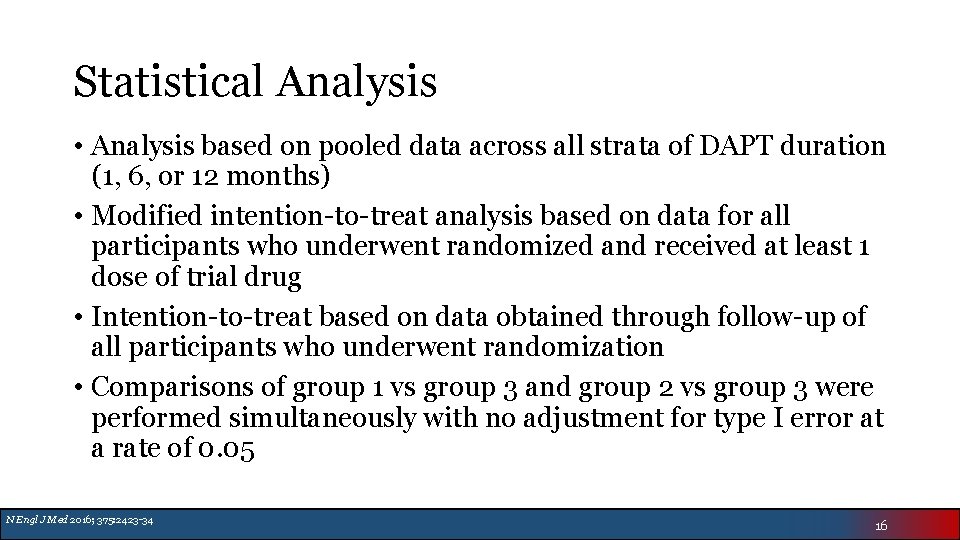
Statistical Analysis • Analysis based on pooled data across all strata of DAPT duration (1, 6, or 12 months) • Modified intention-to-treat analysis based on data for all participants who underwent randomized and received at least 1 dose of trial drug • Intention-to-treat based on data obtained through follow-up of all participants who underwent randomization • Comparisons of group 1 vs group 3 and group 2 vs group 3 were performed simultaneously with no adjustment for type I error at a rate of 0. 05 N Engl J Med 2016; 375: 2423 -34 16
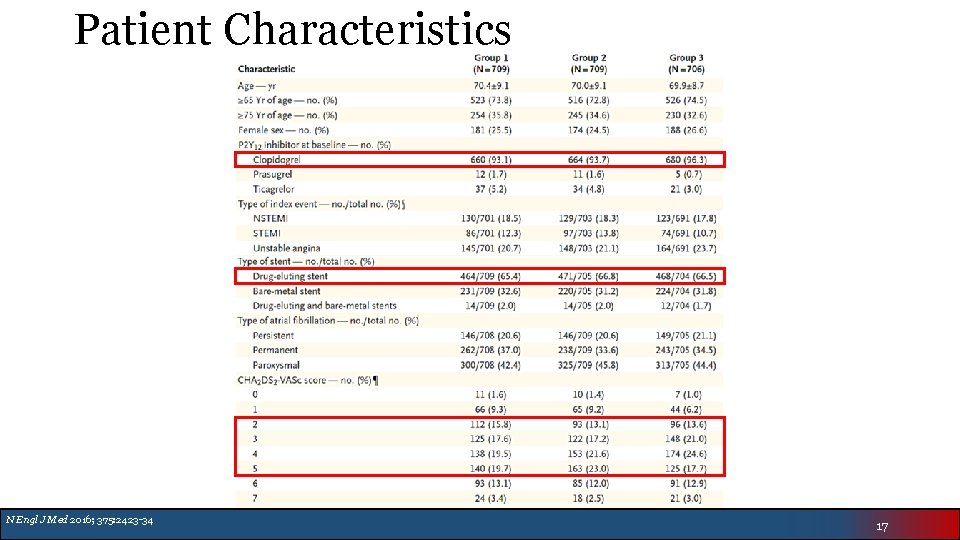
Patient Characteristics N Engl J Med 2016; 375: 2423 -34 17
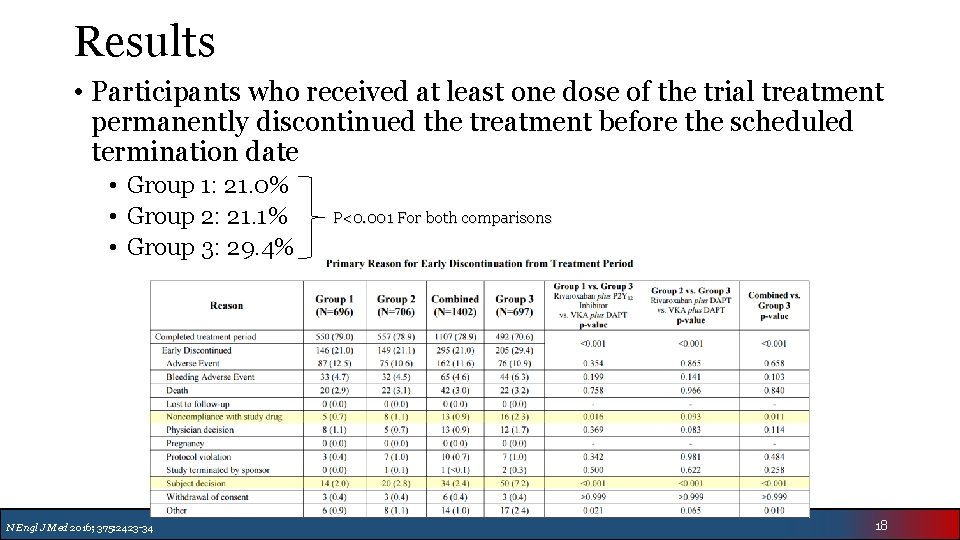
Results • Participants who received at least one dose of the trial treatment permanently discontinued the treatment before the scheduled termination date • Group 1: 21. 0% • Group 2: 21. 1% • Group 3: 29. 4% N Engl J Med 2016; 375: 2423 -34 P<0. 001 For both comparisons 18
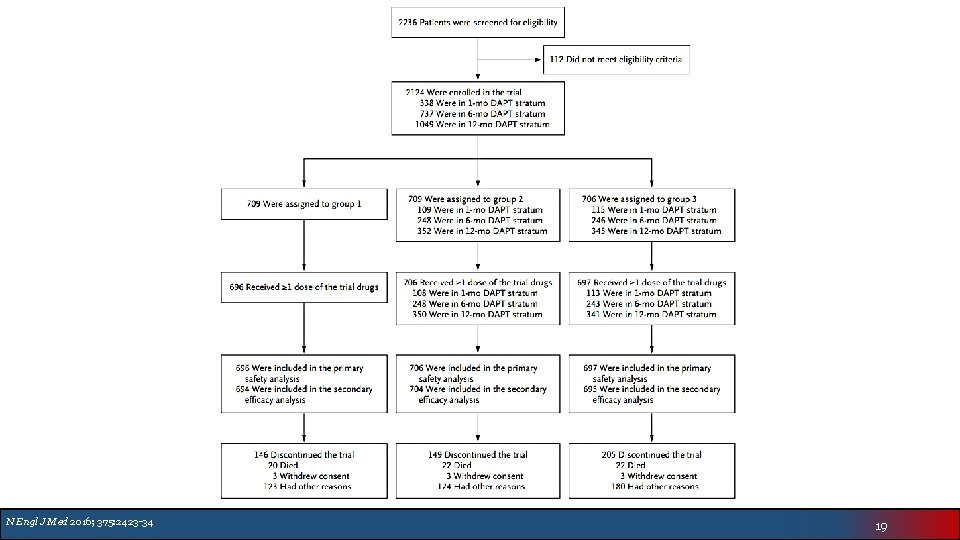
N Engl J Med 2016; 375: 2423 -34 19
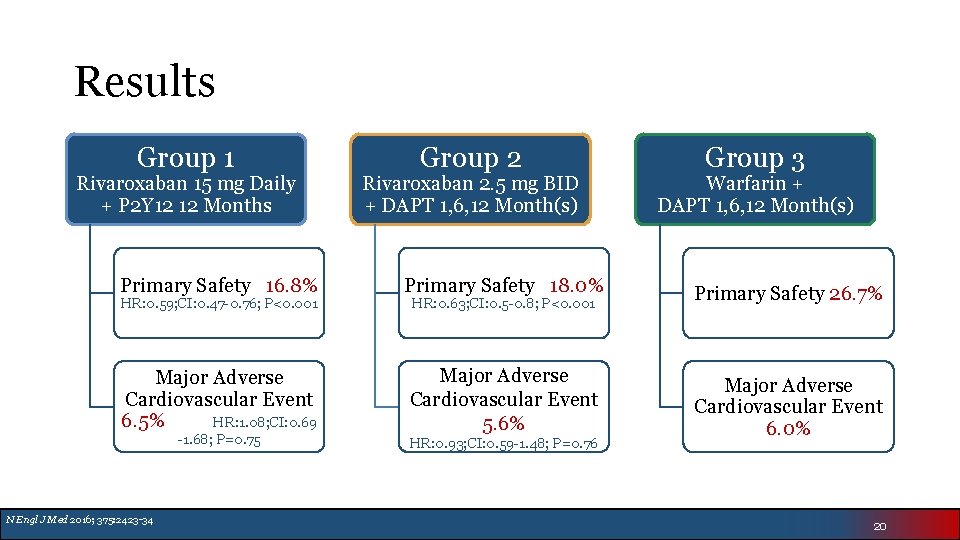
Results Group 1 Rivaroxaban 15 mg Daily + P 2 Y 12 12 Months Group 2 Rivaroxaban 2. 5 mg BID + DAPT 1, 6, 12 Month(s) Group 3 Warfarin + DAPT 1, 6, 12 Month(s) Primary Safety 16. 8% Primary Safety 18. 0% Primary Safety 26. 7% Major Adverse Cardiovascular Event 6. 5% HR: 1. 08; CI: 0. 69 Major Adverse Cardiovascular Event 5. 6% Major Adverse Cardiovascular Event 6. 0% HR: 0. 59; CI: 0. 47 -0. 76; P<0. 001 -1. 68; P=0. 75 N Engl J Med 2016; 375: 2423 -34 HR: 0. 63; CI: 0. 5 -0. 8; P<0. 001 HR: 0. 93; CI: 0. 59 -1. 48; P=0. 76 20
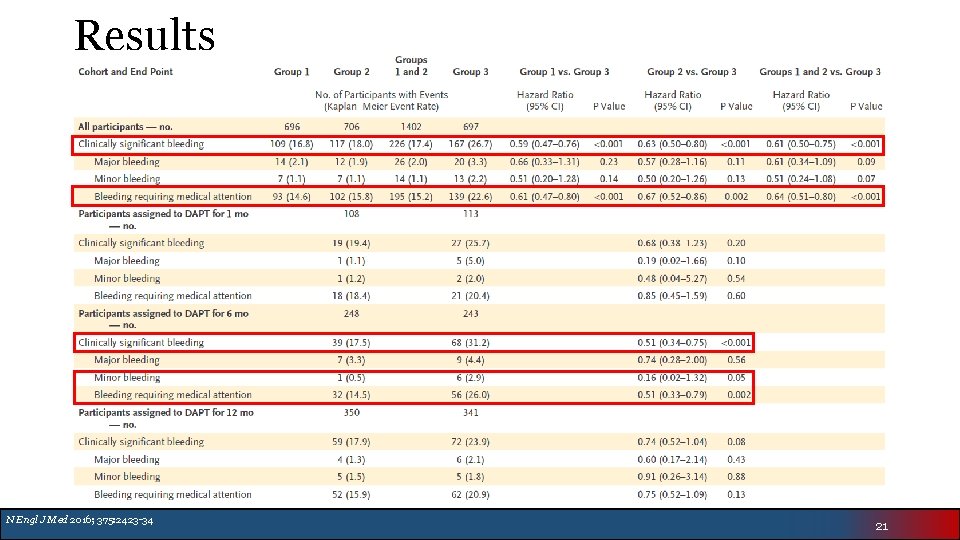
Results N Engl J Med 2016; 375: 2423 -34 21
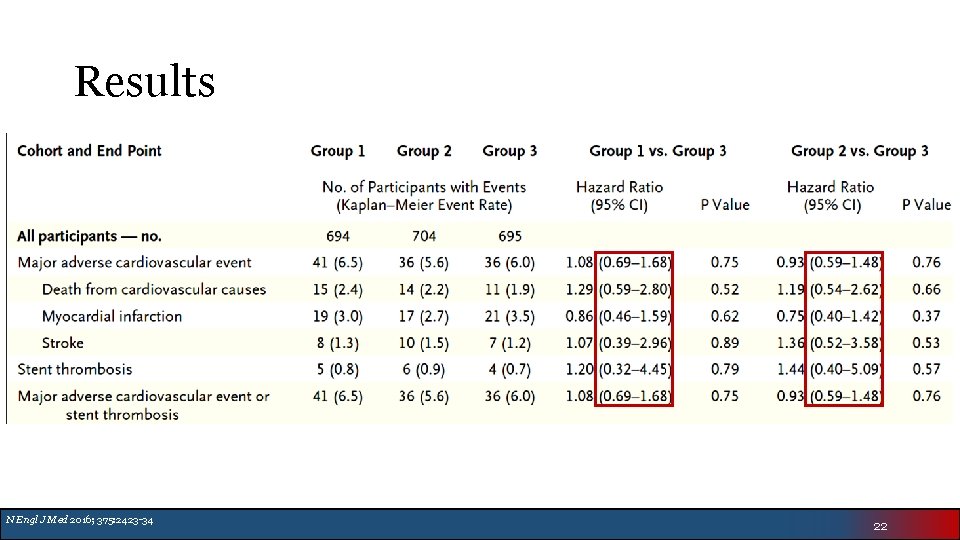
Results N Engl J Med 2016; 375: 2423 -34 22
Authors’ Conclusion • Low-dose and very-low-dose rivaroxaban was associated with lower risk of clinically significant bleeding than standard triple therapy with warfarin • The rates of major adverse cardiovascular events were similar • Broad confidence intervals make efficacy difficult to assess • Cannot truly assess stroke prevention of 15 mg or 2. 5 mg BID rivaroxaban doses N Engl J Med 2016; 375: 2423 -34 23
Critique • Shorter duration of triple therapy (22% of patients) as compared to the WOEST trial (66% of patients) • More reflective of current clinical practice • Limitations • Trial not powered to establish superiority or inferiority of secondary endpoints • Rivaroxaban 15 mg daily dose not currently approved for ACS or AF for patients with normal renal function • Rivaroxaban 2. 5 mg BID currently only approved in Europe for ACS • Overall trial is underpowered and individual efficacy end points within subgroups are even more underpowered • Stratification to 1, 6, or 12 months of DAPT was based on physician choice creating an imbalance of patient characteristics • Broad confidence intervals of efficacy endpoints make it difficult to draw reliable conclusions regarding efficacy 24
Impact on Clinical Practice • Difficult to make definitive recommendations based solely off this study • Recommend stratification strategy similar to ESC 2016 AF guidelines • Shortest duration of triple therapy possible • Important to assess patient risks regarding bleeding vs stroke • Patients at higher risk of bleeding may benefit from lower dose rivaroxaban treatment strategy • Additional Considerations • Tighter INR control with warfarin (2. 0 -2. 5) in high risk patients • Procedural considerations • Stent choice • Glycoprotein IIb/IIIa inhibitor exposure • Ongoing trials • RE-DUAL PCI™ • Dabigatran 150 mg or 110 mg + Clopidogrel or Ticagrelor vs Triple Therapy • AUGUSTUS • Apixaban 5 mg BID ± ASA vs Warfarin ± ASA in addition to a P 2 Y 12 inhibitor for 6 months • ENTRUST AF-PCI • Edoxaban 60 mg daily + P 2 Y 12 vs Warfarin plus P 2 Y 12 + ASA x 1 -12 months 25

Acknowledgements • Journal Club Mentor: • Denise Sutter, Pharm. D, BCPS • Program Director: • Matthew Lillyblad, Pharm. D, BCPS-AQ Cardiology • ACCP Cardiology PRN Journal Club Coordinators: • Genevieve Hale, Pharm. D, BCPS • Ted Berei, Pharm. D, MBA • Zachary Noel, Pharm. D, BCPS 26
- Slides: 26