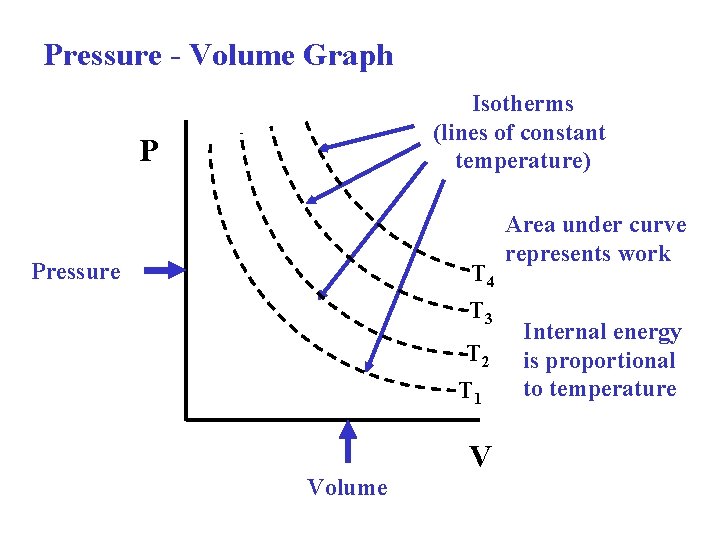
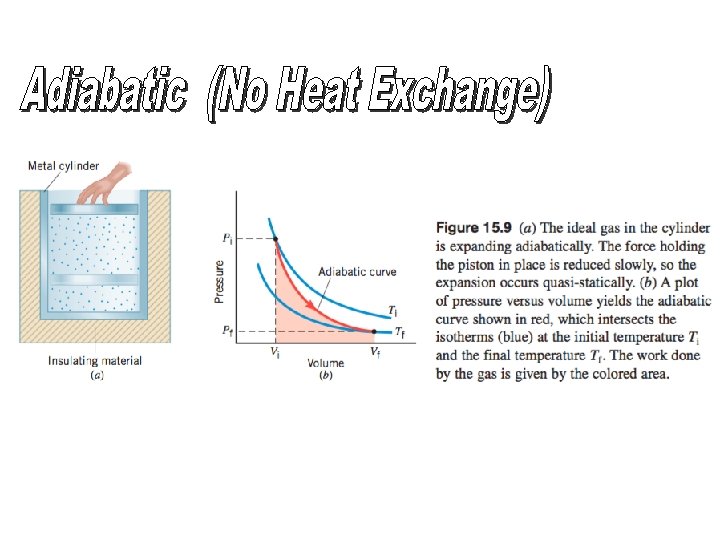
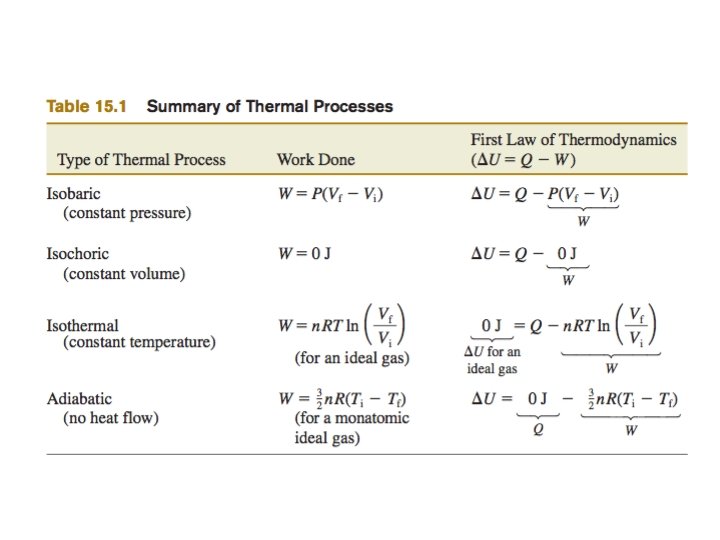
Pressure Volume Graph Isotherms lines of constant temperature

- Slides: 26

Pressure - Volume Graph Isotherms (lines of constant temperature) P Pressure T 4 T 3 T 2 T 1 Volume V Area under curve represents work Internal energy is proportional to temperature


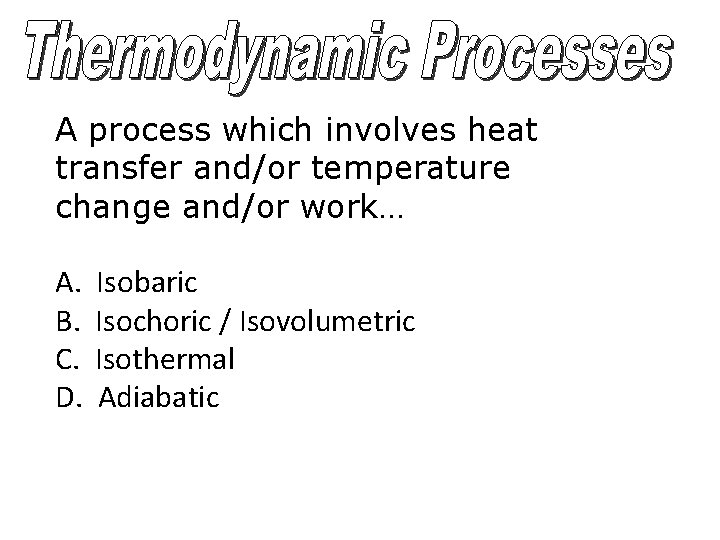
A process which involves heat transfer and/or temperature change and/or work… A. B. C. D. Isobaric Isochoric / Isovolumetric Isothermal Adiabatic

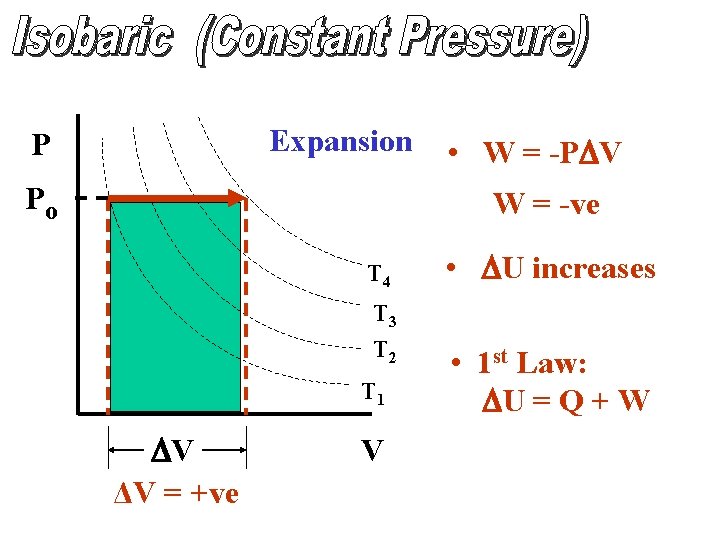
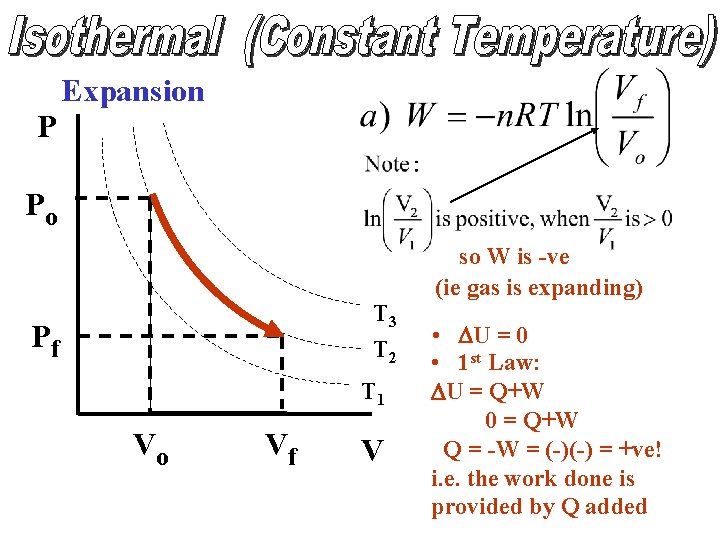
Expansion P Po • W = -PDV W = -ve T 4 T 3 T 2 T 1 DV ΔV = +ve V • DU increases • 1 st Law: DU = Q + W


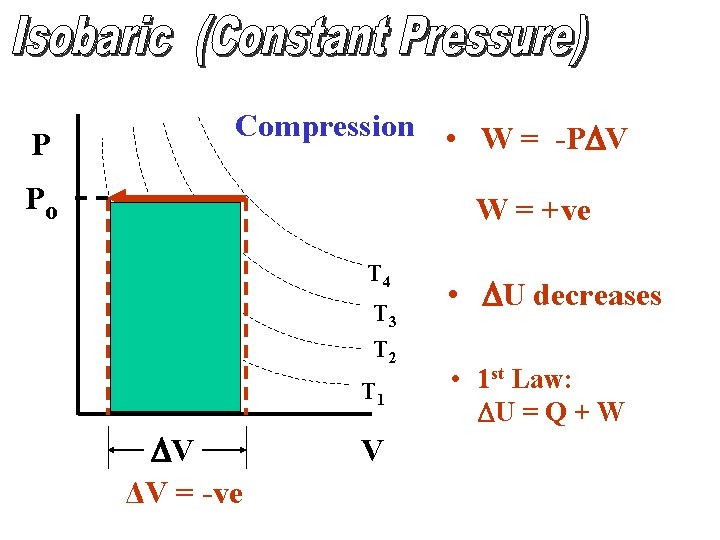
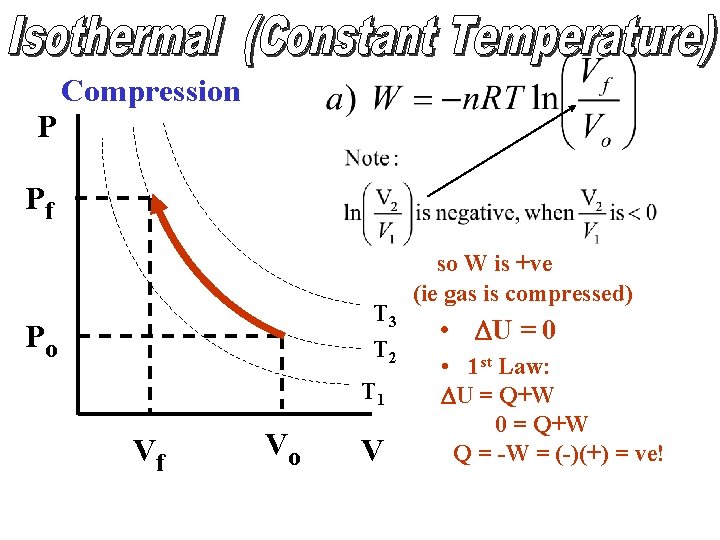
P Compression • W = -PDV Po W = +ve T 4 T 3 T 2 T 1 DV ΔV = -ve V • DU decreases • 1 st Law: DU = Q + W

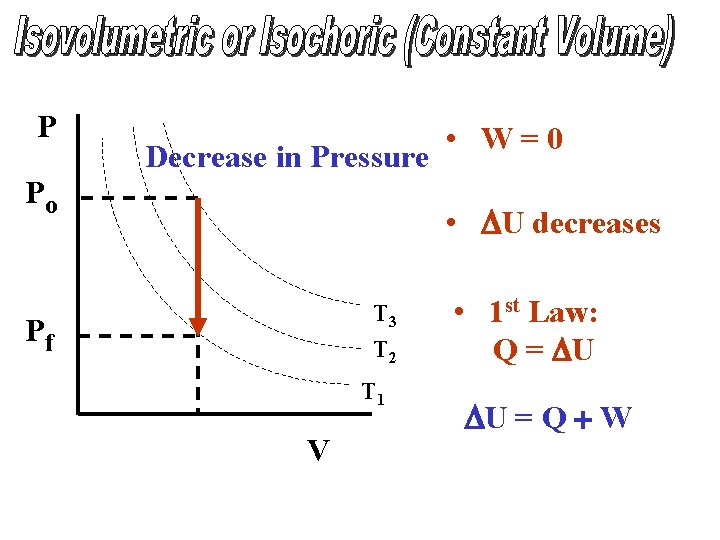
P Decrease in Pressure Po • W=0 • DU decreases T 3 T 2 Pf T 1 V • 1 st Law: Q = DU DU = Q + W

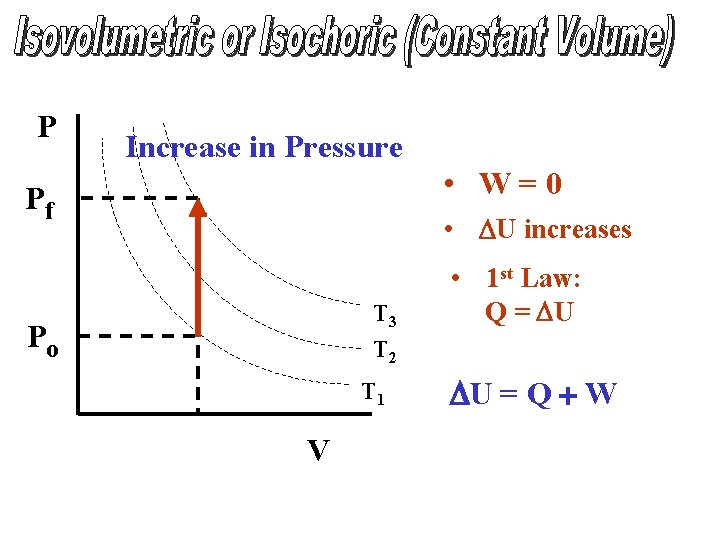
P Increase in Pressure • W=0 Pf • DU increases T 3 T 2 Po T 1 V • 1 st Law: Q = DU DU = Q + W


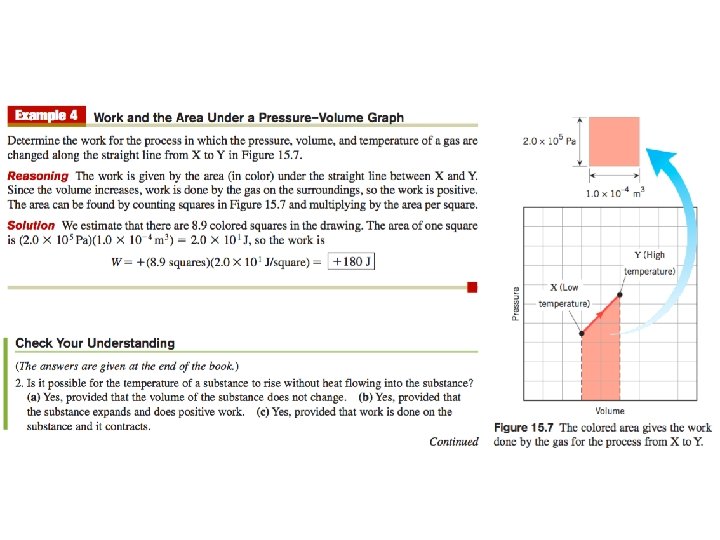
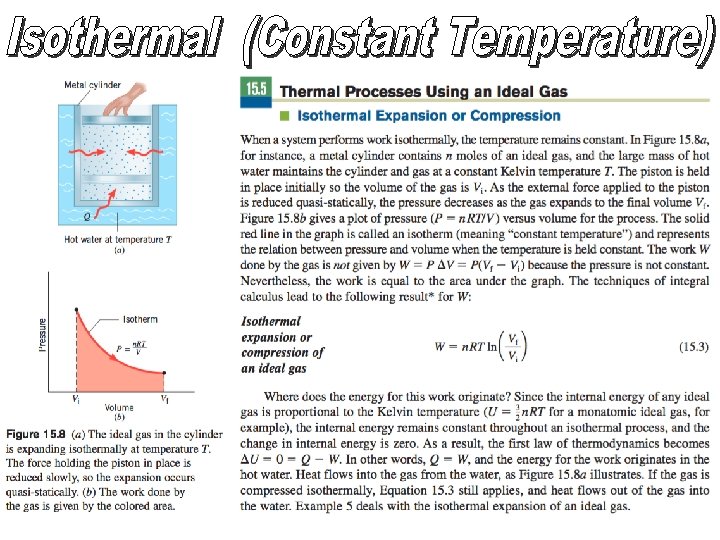
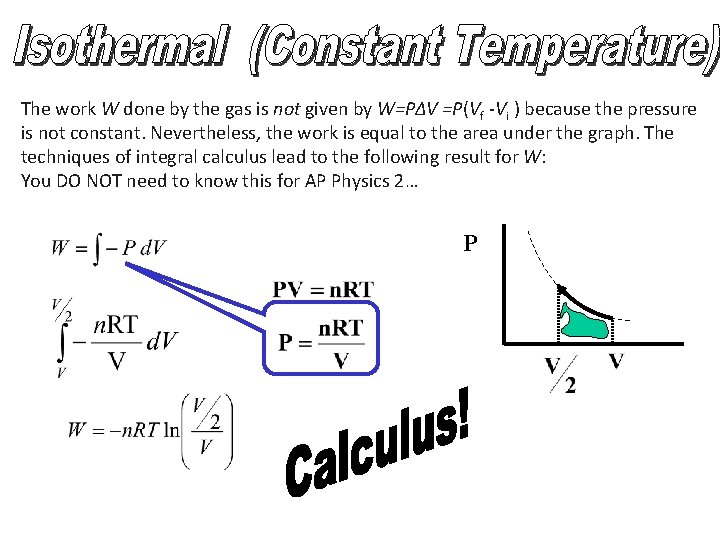
The work W done by the gas is not given by W=PΔV =P(Vf -Vi ) because the pressure is not constant. Nevertheless, the work is equal to the area under the graph. The techniques of integral calculus lead to the following result for W: You DO NOT need to know this for AP Physics 2… P

Expansion P Po T 3 T 2 Pf T 1 Vo Vf V so W is -ve (ie gas is expanding) • DU = 0 • 1 st Law: DU = Q+W 0 = Q+W Q = -W = (-)(-) = +ve! i. e. the work done is provided by Q added

Compression P Pf T 3 T 2 Po T 1 Vf Vo V so W is +ve (ie gas is compressed) • DU = 0 • 1 st Law: DU = Q+W 0 = Q+W Q = -W = (-)(+) = ve!


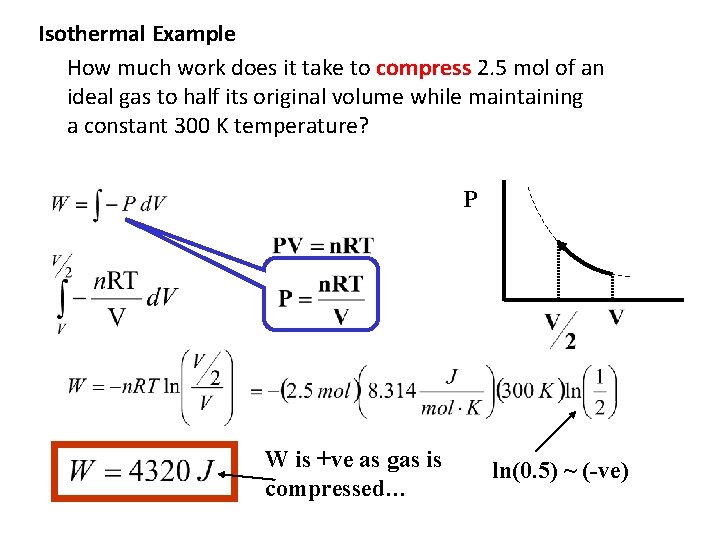
Isothermal Example How much work does it take to compress 2. 5 mol of an ideal gas to half its original volume while maintaining a constant 300 K temperature? P W is +ve as gas is compressed… ln(0. 5) ~ (-ve)

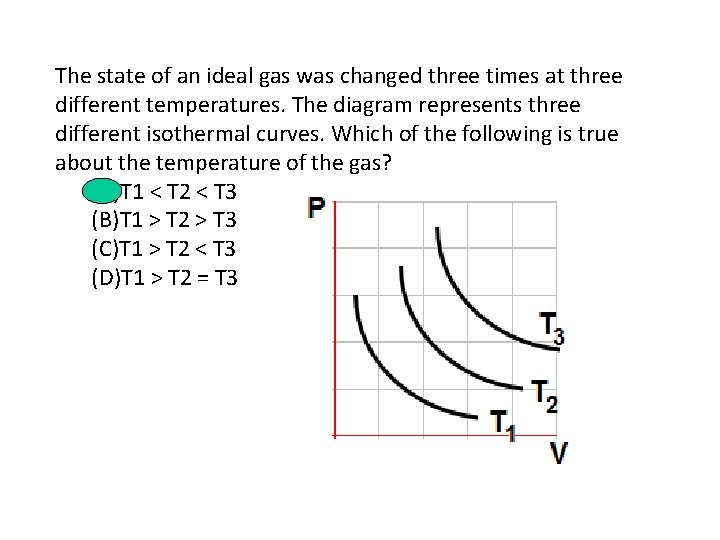
The state of an ideal gas was changed three times at three different temperatures. The diagram represents three different isothermal curves. Which of the following is true about the temperature of the gas? (A)T 1 < T 2 < T 3 (B)T 1 > T 2 > T 3 (C)T 1 > T 2 < T 3 (D)T 1 > T 2 = T 3



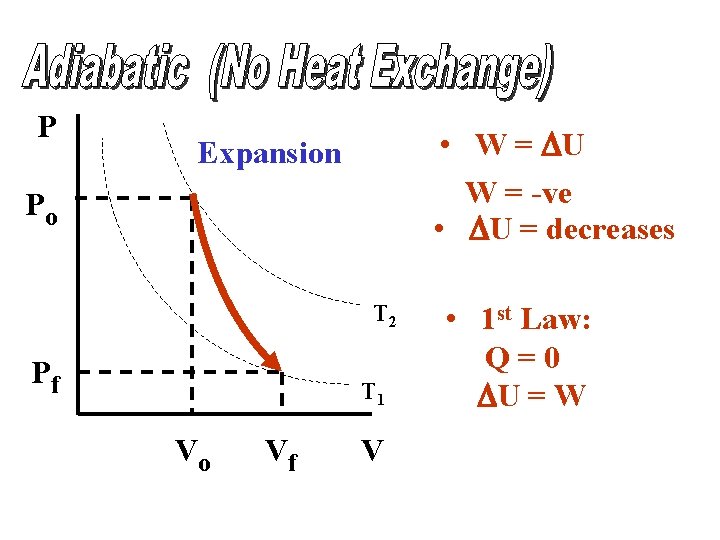
P • W = DU W = -ve • DU = decreases Expansion Po T 2 Pf T 1 Vo Vf V • 1 st Law: Q=0 DU = W

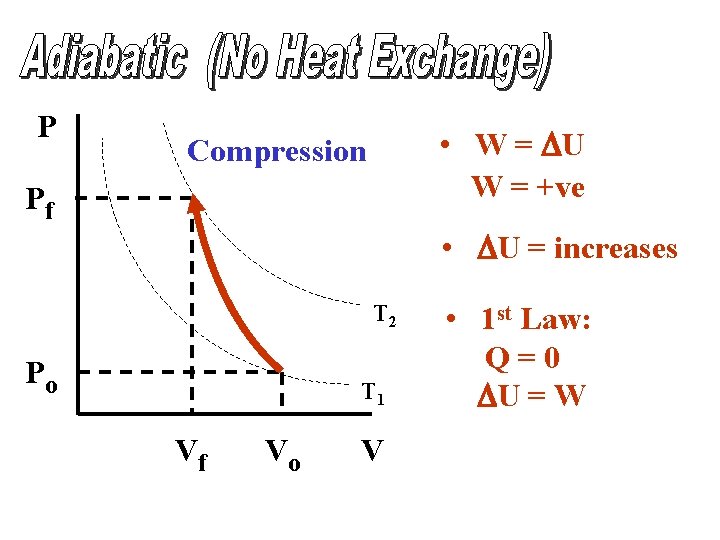
P • W = DU W = +ve Compression Pf • DU = increases T 2 Po T 1 Vf Vo V • 1 st Law: Q=0 DU = W

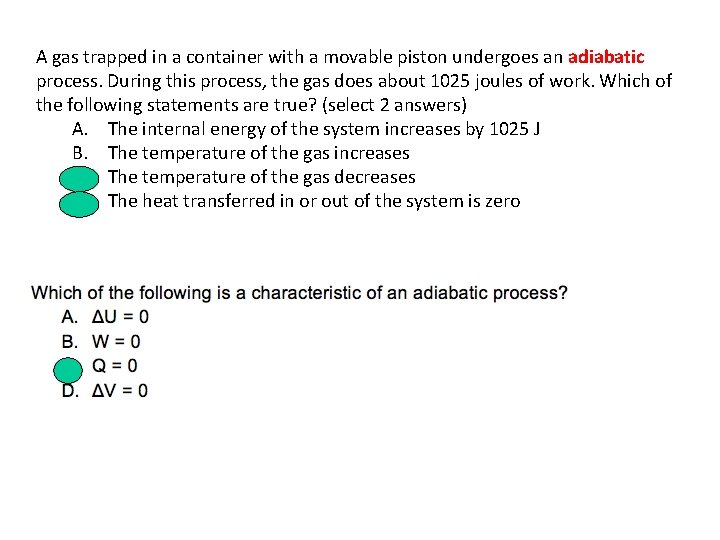
A gas trapped in a container with a movable piston undergoes an adiabatic process. During this process, the gas does about 1025 joules of work. Which of the following statements are true? (select 2 answers) A. The internal energy of the system increases by 1025 J B. The temperature of the gas increases C. The temperature of the gas decreases D. The heat transferred in or out of the system is zero

MC Questions that follow will test your understanding of all 4 thermal processes…




