Pressure measurement From Wikipedia the free encyclopedia Manometer













- Slides: 24

Pressure measurement From Wikipedia, the free encyclopedia • Manometer: a pressure measuring instrument, usually limited to measuring pressures near to atmospheric. The term manometer is often used to refer specifically to liquid column hydrostatic instruments.

Zero reference • Absolute pressure is zero referenced against a “perfect vacuum” (it-the value-is equal to gauge pressure plus atmospheric pressure). • Gauge pressure is zero referenced against ambient air pressure; it-the value-is equal to absolute pressure minus atmospheric pressure. Negative signs are usually omitted; often expressed as “inches of vacuum” or some such. Examples? • Differential pressure is the difference in pressure between two points. Examples of uses?

Zero reference • • Absolute pressure Gauge pressure Differential pressure Are these distinctions: – Meaningful? – Accurate? – Useful? – Explain and discuss

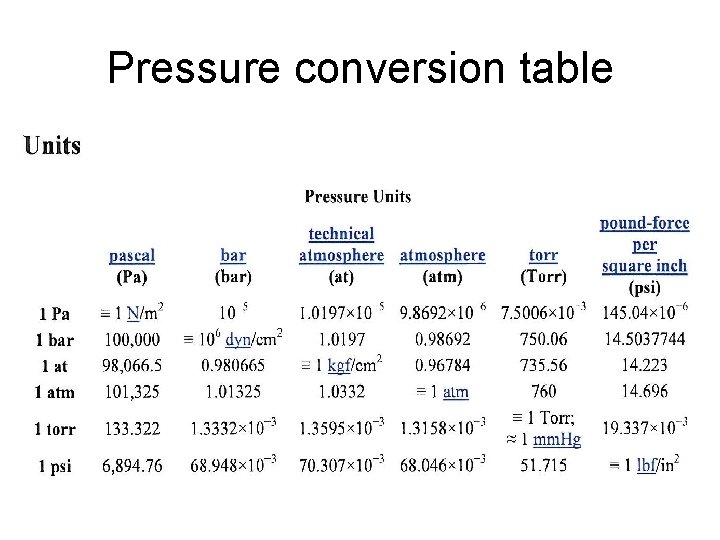
Pressure conversion table

Error? In Wikipedia? ? ? • “In American and Canadian engineering, stress is often measured in kip. Note that stress is not a true pressure since it is not scalar. “ • Really! “kip” stands for kilopounds. Is this a unit of stress or pressure?

Is pressure related to stress? • Pressure is a property of fluids, which, by definition cannot support a shear. • Stress comes in three forms: – Tensile/compressive stresses are related to forces normal to a surface – Shear stresses are in the plane of the surface – The bulk modulus is related to hydrostatic forces (pressure) • Except for the fact that the bulk modulus is measured by applying hydrostatic pressure, stress relates to properties of solids • Stress is not relevant to discussions of pressure

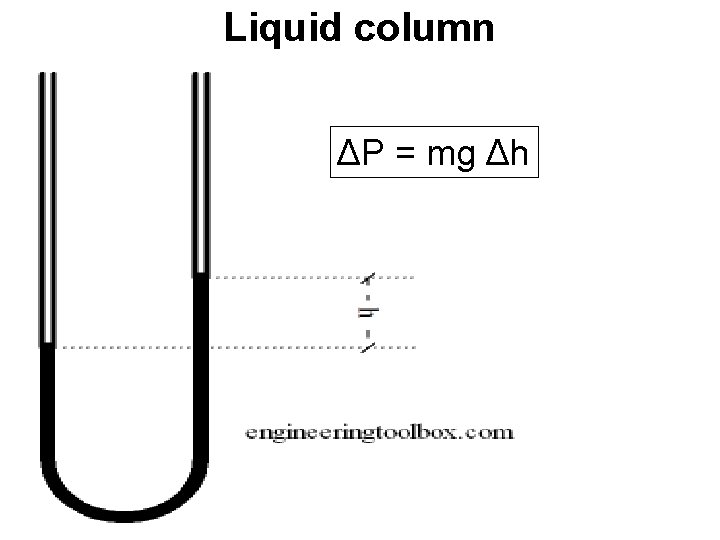
Hydrostatic Gauges • Hydrostatic gauges (such as the mercury column manometer) compare pressure to the hydrostatic force per unit area at the base of a column of fluid. Hydrostatic gauge measurements are independent of the type of gas being measured, and can be designed to have a very linear calibration. They have poor dynamic response. • Why?

Piston • Piston-type gauges counterbalance the pressure of a fluid with a solid weight or a spring. For example dead-weight testers used for calibration and Tire-pressure gauges. • How do you get from spring displacement to pressure? • How do you “calibrate” a tire gauge?

Liquid column ΔP = mg Δh

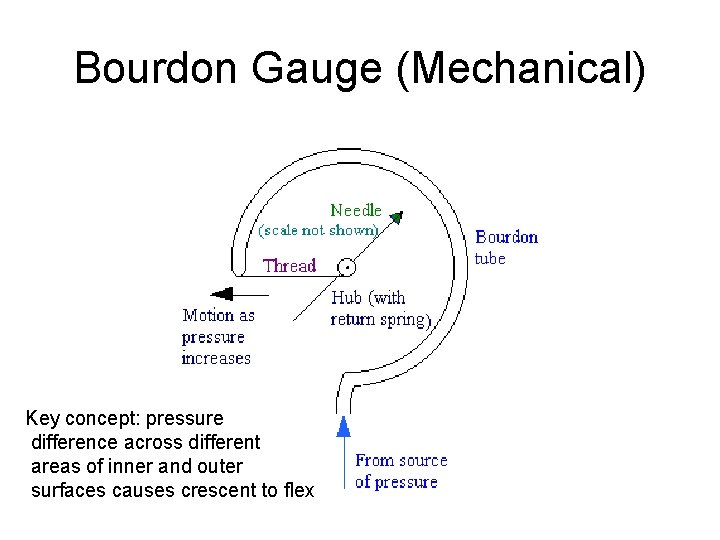
Bourdon Gauge (Mechanical) Key concept: pressure difference across different areas of inner and outer surfaces causes crescent to flex

Diaphragm (Ancient, mechanical) If each element is sealed with a known, fixed internal pressure, flex will depend on pressure change on outside. Stacking amplifies effect!

Diaphragm (modern, capacitance) • resistive (strain gauge) • inductive • capacitive - The deflection of the piston is often one half of a capacitor, so that when the piston moves, the capacitance of the device changes. This is a common way (with proper calibrations) to get a very precise, electronic reading from a manometer, and this configuration is called a capacitive manometer vacuum gauge. • “This is also called a capacitance manometer, in which the diaphragm makes up a part of a capacitor. A change in pressure leads to the flexure of the diaphragm, which results in a change in capacitance. These gauges are effective from 10− 3 Torr to 10− 4 Torr. ” [Nonsense! MKS sells capacitance gages over the range 0. 01 – 155, 000 Torr!] • piezoelectric/piezoresistive

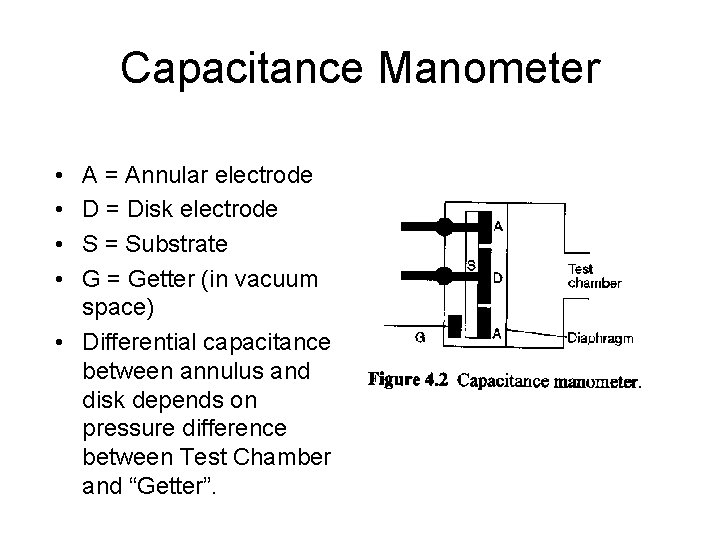
Capacitance Manometer • • A = Annular electrode D = Disk electrode S = Substrate G = Getter (in vacuum space) • Differential capacitance between annulus and disk depends on pressure difference between Test Chamber and “Getter”.

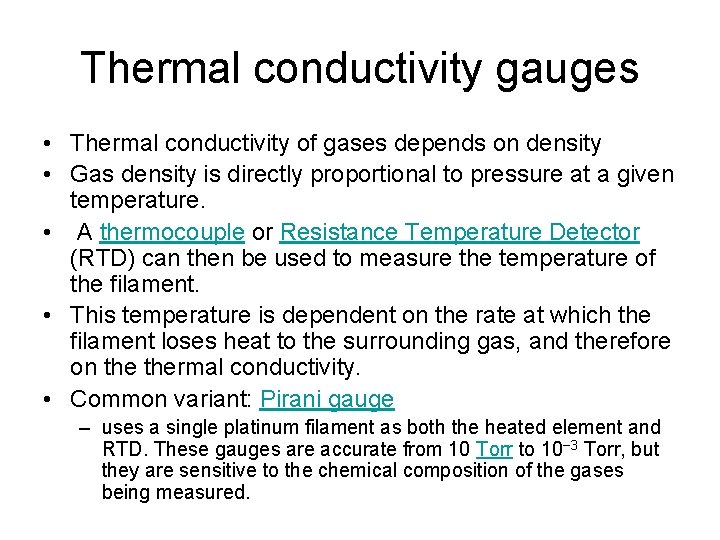
Thermal conductivity gauges • Thermal conductivity of gases depends on density • Gas density is directly proportional to pressure at a given temperature. • A thermocouple or Resistance Temperature Detector (RTD) can then be used to measure the temperature of the filament. • This temperature is dependent on the rate at which the filament loses heat to the surrounding gas, and therefore on thermal conductivity. • Common variant: Pirani gauge – uses a single platinum filament as both the heated element and RTD. These gauges are accurate from 10 Torr to 10− 3 Torr, but they are sensitive to the chemical composition of the gases being measured.

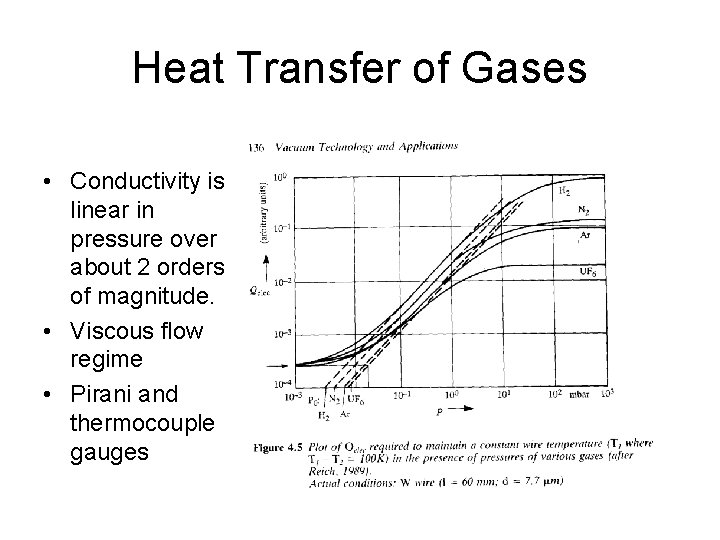
Heat Transfer of Gases • Conductivity is linear in pressure over about 2 orders of magnitude. • Viscous flow regime • Pirani and thermocouple gauges

Ionization gauge • Most sensitive gauges for very low pressures (high vacuums, AKA "hard" vacuums). • Sense pressure indirectly by measuring the electrical ion current produced when the gas is bombarded with electrons. • Ion density will be proportional to gas density. • The calibration of an ion gauge is unstable and dependent on the nature of the gases being measured, which is not always known.

Ionization gauges • Useful range: 10 -10 - 10 -3 Torr (roughly 10 -8 - 10 -1 Pa) • Hot cathode version: an electrically heated filament produces an electron beam. The electrons ionize residual gas molecules. Ion current depends on the number of ions, which depends on the pressure in the gauge. • Cold cathode version: the same, except that ions are produced by electrons from a high voltage electrical discharge.

Making vacuum: pumps • Two kinds: – Displacement • Remove gas from system by putting it somewhere else • Works like a water pump – Entrapment • Remove gas molecules from vapor • “Gas” remains in the system • Works like a garbage can? Better example?

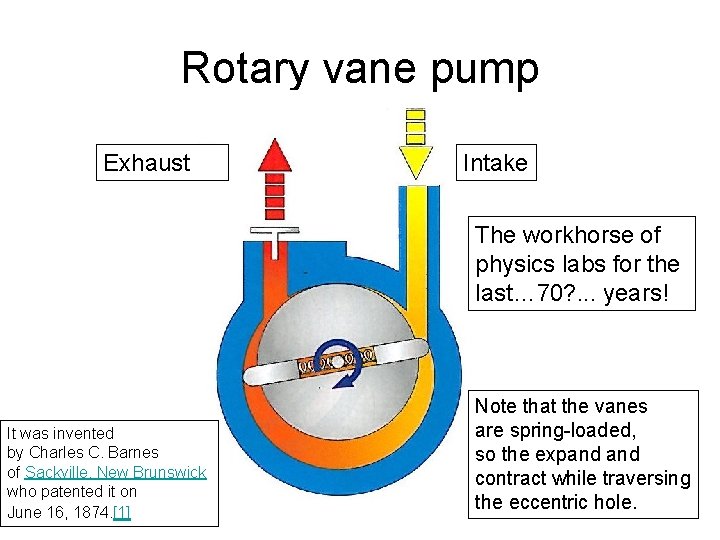
Rotary vane pump Exhaust Intake The workhorse of physics labs for the last… 70? . . . years! It was invented by Charles C. Barnes of Sackville, New Brunswick who patented it on June 16, 1874. [1] Note that the vanes are spring-loaded, so the expand contract while traversing the eccentric hole.

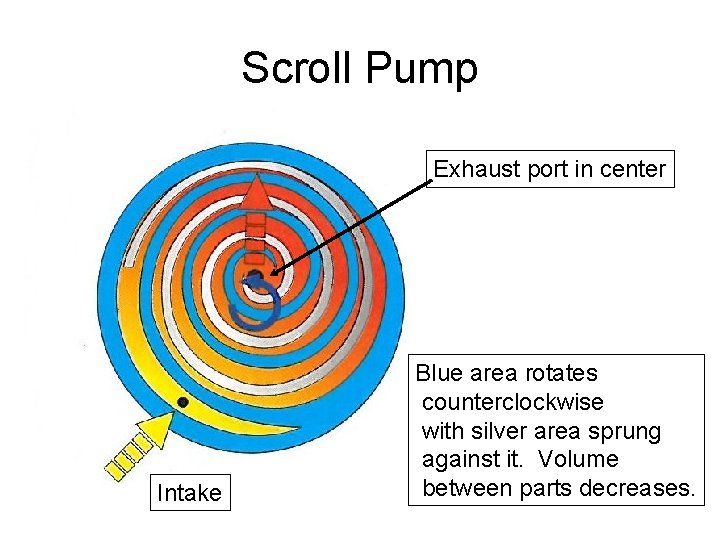
Scroll Pump Exhaust port in center Intake Blue area rotates counterclockwise with silver area sprung against it. Volume between parts decreases.

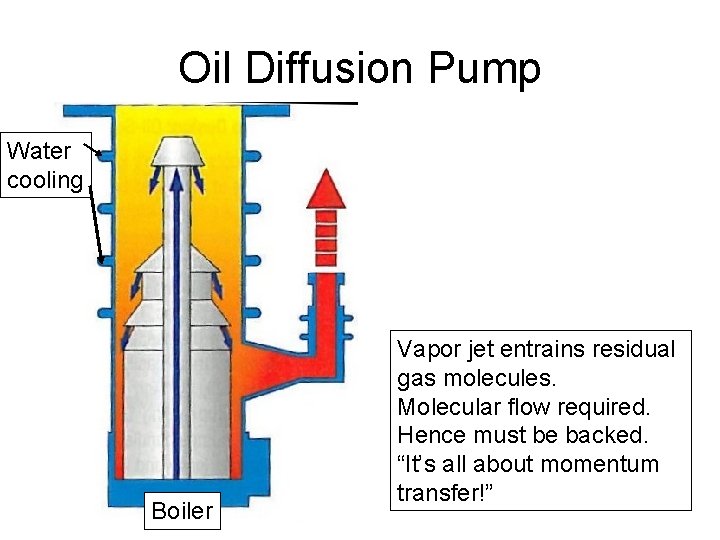
Oil Diffusion Pump Water cooling Text Te xt Boiler Vapor jet entrains residual gas molecules. Molecular flow required. Hence must be backed. “It’s all about momentum transfer!”

Turbomolecular Pump Oilfree Must be backed Vacuum to 10 -7 Torr Expensive Finite lifetime (5 years)

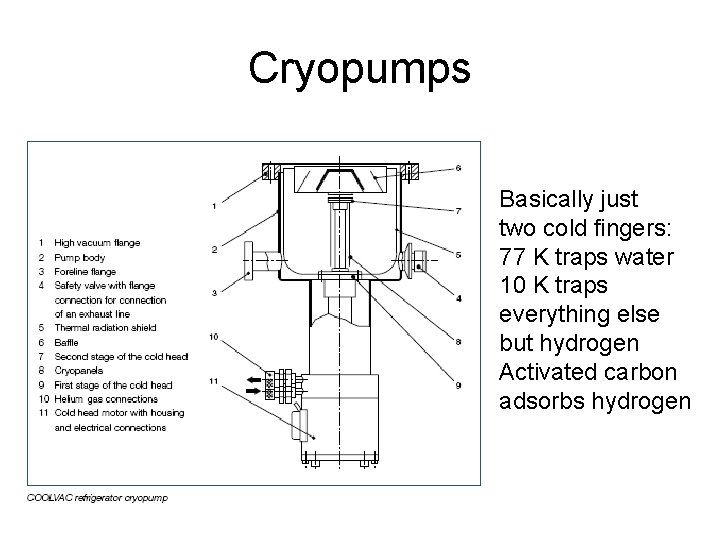
Cryopumps Basically just two cold fingers: 77 K traps water 10 K traps everything else but hydrogen Activated carbon adsorbs hydrogen

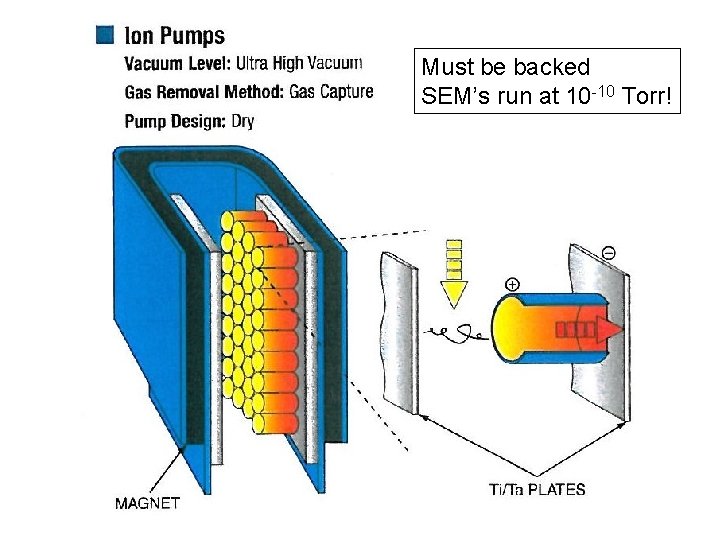
Must be backed SEM’s run at 10 -10 Torr!