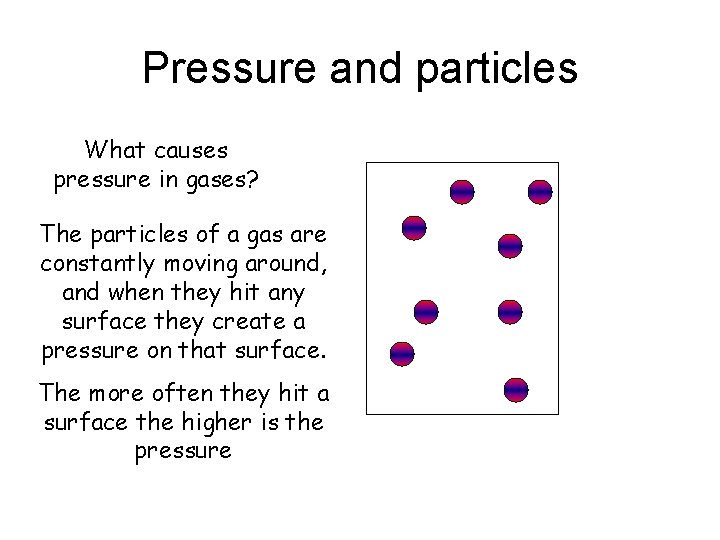
Pressure and particles What causes pressure in gases
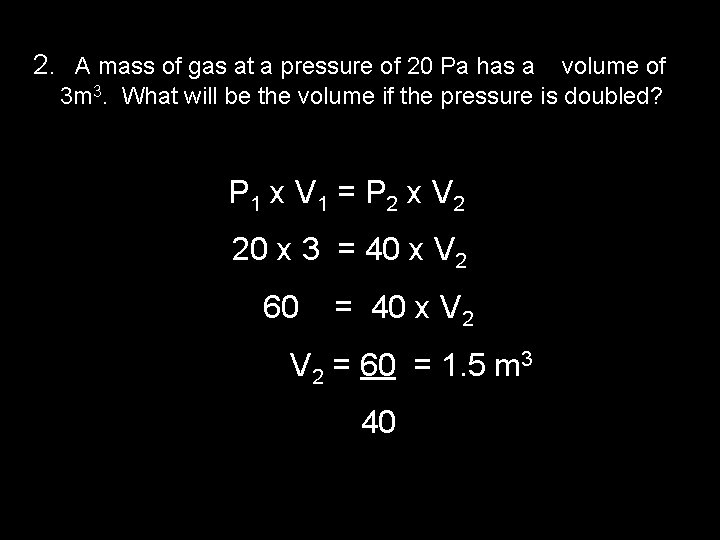


- Slides: 9

Pressure and particles What causes pressure in gases? The particles of a gas are constantly moving around, and when they hit any surface they create a pressure on that surface. The more often they hit a surface the higher is the pressure

Boyle’s Law This law is about what happens to the pressure of a gas when the volume of a container changes and the temperature of the gas remains constant The pressure depends on how often the particles hit the walls of the container The more often, the higher the pressure, the less often the lower the pressure

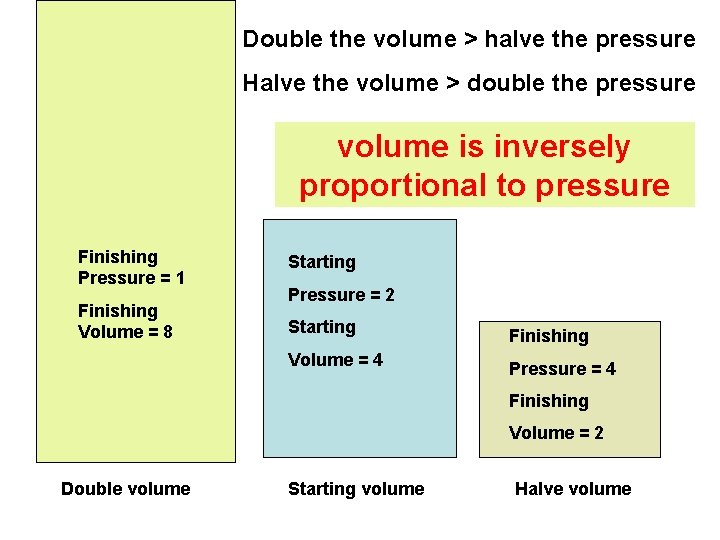
Double the volume > halve the pressure Halve the volume > double the pressure volume is inversely proportional to pressure Finishing Pressure = 1 Starting Finishing Volume = 8 Starting Finishing Volume = 4 Pressure = 2 Finishing Volume = 2 Double volume Starting volume Halve volume

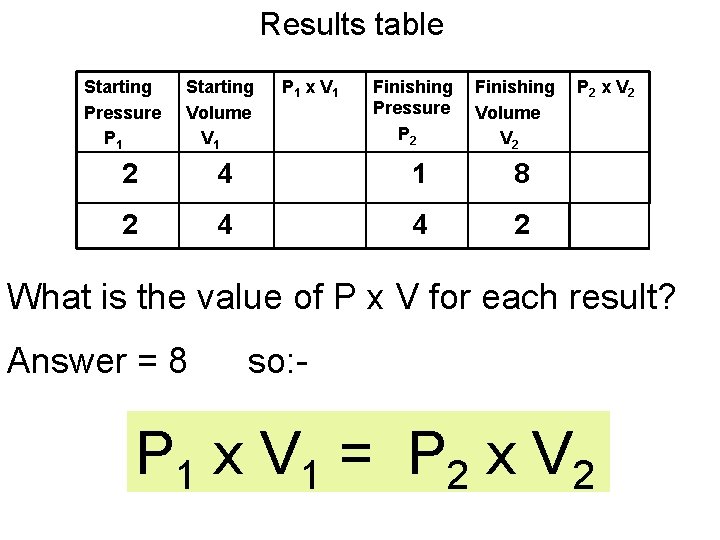
Results table Starting Pressure P 1 Starting Volume V 1 P 1 x V 1 Finishing Pressure P 2 Finishing Volume V 2 P 2 x V 2 2 4 8 1 8 8 2 4 8 4 2 8 What is the value of P x V for each result? Answer = 8 so: - P 1 x V 1 = P 2 x V 2

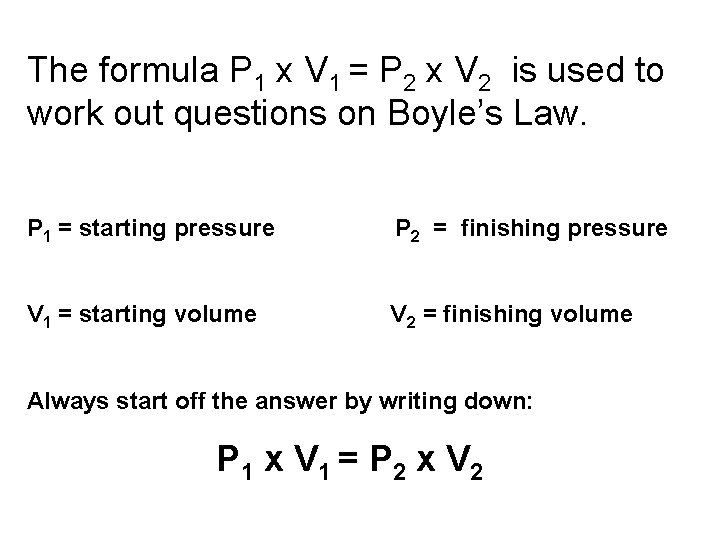
The formula P 1 x V 1 = P 2 x V 2 is used to work out questions on Boyle’s Law. P 1 = starting pressure P 2 = finishing pressure V 1 = starting volume V 2 = finishing volume Always start off the answer by writing down: P 1 x V 1 = P 2 x V 2

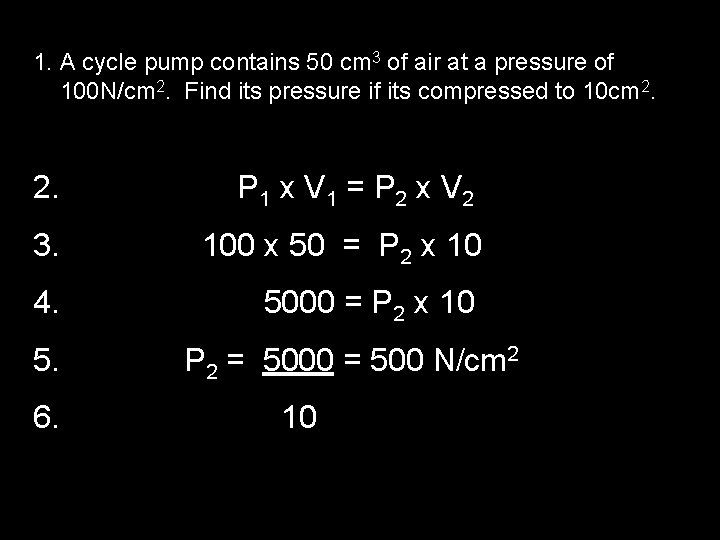
1. A cycle pump contains 50 cm 3 of air at a pressure of 100 N/cm 2. Find its pressure if its compressed to 10 cm 2. P 1 x V 1 = P 2 x V 2 3. 100 x 50 = P 2 x 10 4. 5000 = P 2 x 10 5. 6. P 2 = 5000 = 500 N/cm 2 10
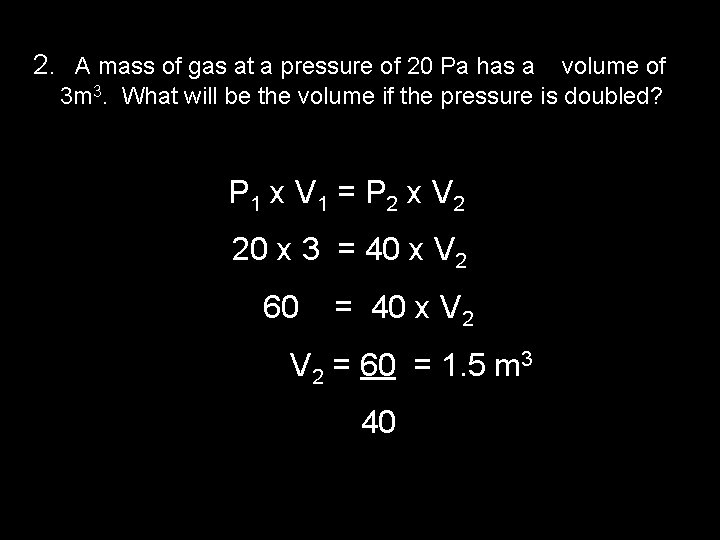
2. A mass of gas at a pressure of 20 Pa has a volume of 3 m 3. What will be the volume if the pressure is doubled? P 1 x V 1 = P 2 x V 2 20 x 3 = 40 x V 2 60 = 40 x V 2 = 60 = 1. 5 m 3 40

3. A balloon contains 8 litres of air at a pressure of 5 Pa. What is the new pressure of the balloon if its volume is decreased to 5 litres? P 1 x V 1 = P 2 x V 2 5 x 8 = P 2 x 5 40 = 5 x V 2 = 40 = 8 Pa 5

4. 100 m 3 of air is at a pressure of 75 N/m 2. If the volume of air is reduced to 30 m 3, what is the new pressure? P 1 x V 1 = P 2 x V 2 75 x 100 = P 2 x 30 7500 = P 2 x 30 P 2 = 7500 = 250 N/m 2 30