PRESENTATION Mission Vision Mission We at QLS provide






















- Slides: 23

PRESENTATION

Mission & Vision Mission We at QLS provide Contracted clinical research services in the pharmaceutical sector of ensured compliance, in targeted international markets, through a team of committed experts and we strive to be long-term trusted partners of major innovative health organisations Vision To achieve leading presence with local expertise in an expanding geography of international CRO markets, through a network of selected partners, developing top professionals and providing cutting edge infrastructure. Excellence in Clinical Research : Our commitment

Our Core Values Quality is our primary value. We ensure quality in our compliance in processes, credibility of data and excellence of deliverables. Customer Focus Our management, processes and people are aligned towards satisfying the needs of our customers. We are dedicated to service excellence that gains us customer continuity. Knowledge We acquire knowledge in order to lead in our field. We share our knowledge with our people and our customers. Commitment We are committed to our mission and to our stakeholders. Our commitment is a promise that we will always perform to the highest of their expectations. Trust We deliver what we promise with honesty and integrity, thus earning the trust that fosters long term relationships. Excellence in Clinical Research : Our commitment

Quest - A Preferred Partner of Choice Speed We believe in speeding up your projects to the next milestone. The faster our customers reach of their milestone, the faster a compound can be advanced to the next stage. Quality We are very dedicated about what we do and want our name to stand for providing quality data. We are determined to be an example that industry aspires to follow. Customer Focus We try and build long lasting, collaborative relationships with our customers and work with them as partners in the clinical development of their products. Disciplined Timelines Importance of time is a mantra followed by our company and we strictly adhere to our commitments. Excellence in Clinical Research : Our commitment

Overview v Established in Year 2004 having 22, 000 Sq. ft area. v Central Headquarters at Chennai, India. v Facilities in 2 Cities (Chennai, Kandla - Gujarat) and more than 100+ professionals operating. v Fully Compliant with ICH GCP, ICMR, and Schedule – Y Guidelines. v Combined Clinical bed Strength of 240, supported by 12 LCMS/MS machines, with more than 150 + validated analytical methods. v > 700 Man years experience. v More than 10, 000 active study participants in our database. v Over 90% business repeat business. Excellence in Clinical Research : Our commitment

Overview… Experience: v 12 + years of Biopharmaceutical experience for Global clients. v 40 + Pharma and Biotech Sponsors all over the globe. v 500 + successful clinical studies for various regulatory submissions. Consistency: v Less than 3% attrition YOY. v More than 80% Repeat Business. Global Footprints : v Operations : India, Russia, Thailand. v Offices in India, USA. Excellence in Clinical Research : Our commitment

Global Regulatory Accreditations - Milestones 2016 2015 2014 2013 2011 UK-MHRA: March. US-FDA: June UK-MHRA: March. Malaysia-MOH: April US-FDA: June DCGI: May US-FDA: October Turkey-MOH: June DCGI: October US-FDA: June DCGI: August US-FDA: November US-FDA: December 2008 US-FDA: May 2006 DCGI: January Excellence in Clinical Research : Our commitment

What we offer @ Quest PK / PD studies Clinical Research v Bioequivalence / Bioavailability studies v PK / PD Studies in patients v Pharmacokinetic / Pharmacodynamic studies in Healthy subjects v Feasibility Studies v Postmenopausal healthy / Patient Population studies. v Study Design and Protocol Writing v Drug – Drug Interaction studies. v Proof of Concept studies - Dermatology v Protocol to Report v Project Management v Final submission Report (e. CTD / ICH E 3) v Clinical end point BE studies v Project Planning v Regulatory Approvals v Design & Development of CRF / e. CRF v Project Management v Site/Investigator Identification and Selection v Site Monitoring v Site Management v Quality Assurance/Audit Services v Global Clinical Trial (Multicentric, multicountry) Excellence in Clinical Research : Our commitment

Offerings @ Quest Life Sciences. . Biostatistics & Medical Writing Stand Alone Services v Sample Size and Power Calculation v Regulatory Services. v Medical writing and literature search v Central Reference Lab Services at Kandla -Gujarat. v Providing Statistical Inputs during Protocol Designing v Bio Analytical Services. v Randomization v Quality Assurance. v Statistical Analysis Plan (SAP) v Pre-Clinical - Russia. v Biostatistical & Medical Writing. v SAS Programming and Validation v Interim Analysis v Statistical Report Excellence in Clinical Research : Our commitment

Quest Credentials Regulatory Inspections @Quest Life Sciences v Successfully completed over 34 regulatory audits by Indian and International regulatory bodies v Completed 50+ National and International sponsor audits X 2 UK MHRA X 9 US FDA X 5 Quest Life Sciences Philippines BFAD Oman USFDA Russia Malaysia Australia X 1 Turkey MOH Applied Malaysia BFPK Philippines X 7 Excellence in Clinical Research : Our commitment

Clinical Pharmacology Chennai v 80 Beds arranged in 2 study areas. v sq. ft area. v 2 Intensive Care Units with cardiac monitoring. v Audited &/or accredited by US FDA, UK MHRA, India (DCGI), and v World-class infrastructure to support clinical activities. v 8 LC-MS/MS for bioanalysis. v Capacity to handle approx. 200 Clinical dosings / month. v Capability to analyze approx. 6, 500 samples / month. Excellence in Clinical Research : Our commitment

Clinical Pharmacology Kandla - Gujarat v 160 Beds arranged in 5 study areas. v Intensive Care Units with cardiac monitoring. v Dedicated Clinical Reference Lab. v 4 LC-MS/MS for bioanalysis. v Independent Project Management Group. Excellence in Clinical Research : Our commitment

Clinical Reference Laboratory at Quest Care, Kandla v Testing @ Quest Care v Hematology and Coagulation Assays v Biochemistry v Clinical Pathology v Immunoassays v Histopathology v NABL (National Accreditation Board for Testing and Calibration Laboratories) accredited laboratory v Services for Clinical Trials having multiple sites & CROs (Stand Alone Services also offered). Excellence in Clinical Research : Our commitment

Bioanalytical Lab v 12 LC-MS/MS (SCIEX API 2000, 3000, 4000, Thermo Vantage) v 150 + validated assays. v Quantitative analysis of parent drugs and their metabolites in a variety of biological matrices. v Solid Phase Extraction Systems. v Nitrogen Evaporators. v Capability to analyse > 12, 000 samples/month. Excellence in Clinical Research : Our commitment

Expertise (Formulations & Therapeutic Areas) Formulations Therapeutic Areas v Oral Solids – Capsules, Tablets, Softgels, Granules, ODT, ODP, Modified Release v Pioneers in oncology v Liquid Orals v Parenterals v Transdermal patches v Topical Gel v Cream v Inhalers v Nasal Sprays v Antidepressant Novel Techniques and Dosage Forms Insulin clamp studies v Antiepileptic Nasal Spray v Antiretroviral Rectal Spray v Hormone Transdermal Patch v Hypoglycemic agents v Lipid lowering agents Narcotic Implants v Muscle relaxants Gums and Lozenges v NSAIDs Opthalmics v Narcotics v Oral contraceptive v Psychotropic agents Antimicrobial Kill rate Biosimilars Excellence in Clinical Research : Our commitment

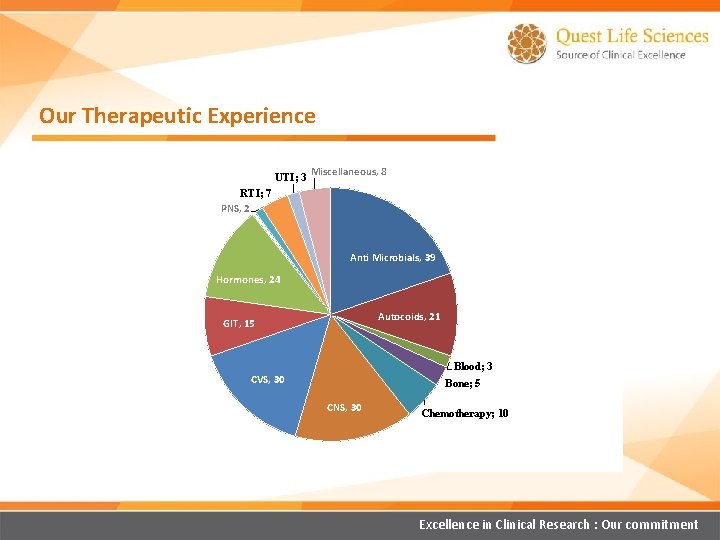
Our Therapeutic Experience UTI; 3 Miscellaneous, 8 RTI; 7 PNS, 2 Anti Microbials, 39 Hormones, 24 Autocoids, 21 GIT, 15 Blood; 3 Bone; 5 CVS, 30 CNS, 30 Chemotherapy; 10 Excellence in Clinical Research : Our commitment

Quest Quality Systems v A 10 member dedicated team reporting to QA Head. v Quality Control (QC) Procedures in place apart from Quality Assurance (QA) Procedures. v All activities are subjected to rigorous review for Quality Assurance including v Clinical v Bio-analytical v PK / Stats / Reporting v Internal Audits by QA team for Area Specific SOP compliance. v In-house capabilities to perform Site Audits, Systems / Process Audit, Vendor Audit. v Document Audits (Protocol, Clinical Study Report & essential Clinical Trial documents). v All QA systems are compliant with all applicable local & international regulations. Excellence in Clinical Research : Our commitment

Quest Clinical Experience and Capabilities v Summary Protocol / Synopsis v Study Feasibility Analysis v Study Design v Medical Writing and Protocol Development v Project Planning and Setup v Regulatory Approvals v Design & Development of CRF / e. CRF v Site/Investigator Identification and Selection v Site Management v Site Monitoring v Project Management v Clinical Data Management v Clinical Study Reports v Quality Assurance / Audit Services Excellence in Clinical Research : Our commitment

Phase Trials (Phase II - IV) v Phase 2 -4 Clinical Trials v > 100 sites v > 150 Investigators v Oncology, CNS, Cardiology, Gastrointestinal, Orthopedic, Ophthalmology, Endocrinology & Metabolism v 505(b)(2) Clinical Studies. v Clinical End Point Studies. v Patient Population PK Studies, Excellence in Clinical Research : Our commitment

Support Teams at Quest v Project Management Group (PMG) v QA Department : 10 experienced personnel to maintain Quality of the highest standards. v HR : A team of 2 people in the managing all the HR functions. Validation : QLS has a dedicated in-house instrument engineer for Validation and the maintenance of Scientific equipment. v Pharmacy: Each of the Clinical Units of QLS and Quest Care has Dedicated Pharmacists for the IMP Management Excellence in Clinical Research : Our commitment

Chemech Laboratories Ltd. , v End- end solution for our clients – FR&D to BA/BE studies v Includes Contract R&D, Tech Transfer and Manufacturing v A list of more than 37 products developed for EU market v Current R&D/ Manufacturing in Mumbai Excellence in Clinical Research : Our commitment

Why Select Quest Life Sciences v Complete service / solution provider with experienced Clinical research professionals and Excellent infrastructure. v QLS capability to harness post menopausal subjects : Special Expertise. v QLS capability to include female subjects in BE trials at no extra cost. v QLS capability to harness patient BE studies involving anti cancer drugs. v QLS capability to partner sponsors in drawing up clinical development programme and execute the v Initial studies till critical mass data is generated for regulatory / outsourcing. v Proven track record from International Regulatory Bodies. v Teams with good professional Experience and expertise in various Therapeutic segment. v Fast and excellent Turn around time. v Competitive Pricing as compared to Industry Peers. Excellence in Clinical Research : Our commitment

Corporate Headquarter Quest Life Sciences P Ltd SDF III, MEPZ Chennai - 600 045, India Mr. T. S Jaishankar Managing Director jai@questlifesciences. com Dr. Gayathri Siva Kumar Director gayathri@questlifesciences. com