Prequel Some Useful Information Everything you need to























- Slides: 30

Prequel: Some Useful Information Everything you need to know is on the Web at: www. fda. gov Hint: If you are not happy with “sort by relevance” Switch the search tool to “sort by date. ”

The Good Review Management Practice Guidance -- A Statistical Reviewer’s Perspective Steve Wilson, Dr. P. H. , CAPT USPHS Deputy Director, Division of Biometrics II, CDER/FDA ASA Biopharmaceutical Section -FDA/Industry Workshop Marriott Wardman Park, Washington, DC September 16 , 2005

Disclaimer Views expressed in this presentation are those of the speaker and not, necessarily, of the Food and Drug Administration.

Acknowledgements • • Dave Christiansen Ruthie Davi Ed Nevius ADa. M Workgroup

Outline • Statistical Review • Good Review Management Practice Guidance • Time for Change – Pre-NDA Statistics Meeting – Meta-Data Standards for Analysis – Statistical Guidance

Statistical Review • Assess quality / completeness of data • Assess compliance with protocol / blinded analysis plans -- conduct of the study • Check appropriateness of statistical models and conclusions • Verify results reported in the NDA • Answer new review-related questions • Examine individual patient experience during clinical trial

A Review Tale

New Guidance for Review Staff and Industry Good Review Management Principles and Practices for PDUFA Products U. S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) April 2005 Procedural

GRMP: Operational Principles • The foundation for good review management is created during product development. • The applicant is responsible for submission of a complete marketing application to maximize the efficiency of the review process and reduce the need for multiple cycle reviews. • Effective and efficient management of the review process is primarily an FDA responsibility.

GRMP : Operational Principles (cont. ) • Active applicant involvement is important during the review process. • Good review management increases first cycle approvals. • Effective and timely communication between the FDA and applicants enhances the review process

GRMP: Fundamental Values • • Quality Efficiency Clarity Transparency Consistency Mom Apple Pie No Surprises

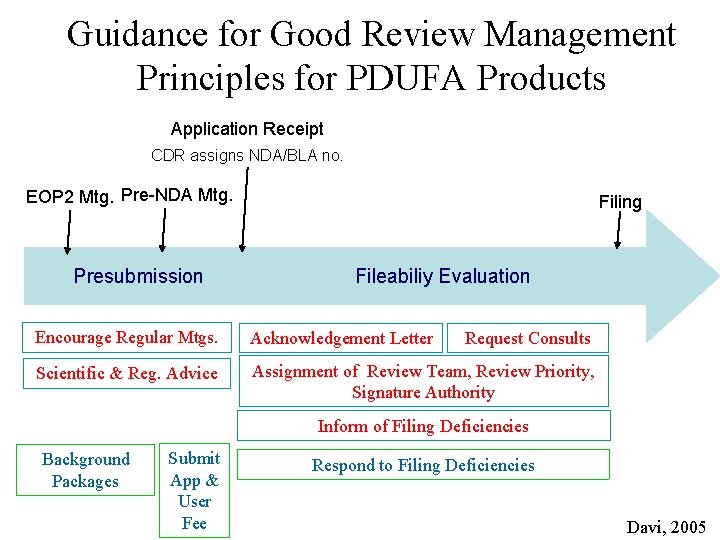
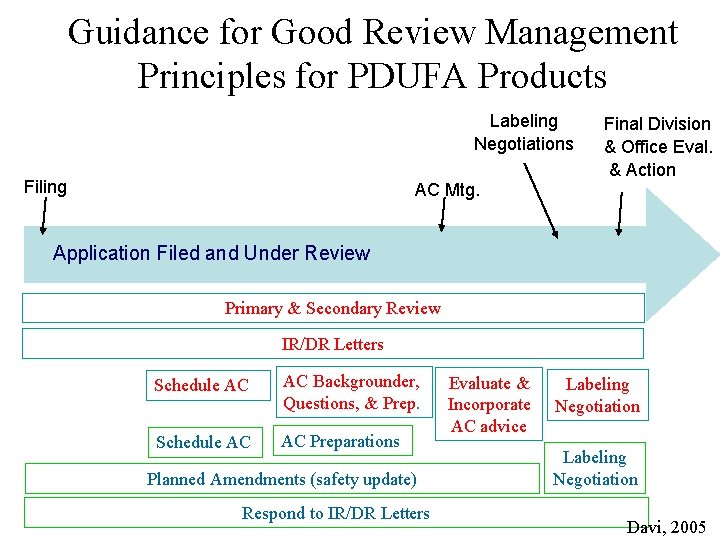
GRMP: FDA Reviewing Steps • • • Application completeness Pre-submission Application receipt Filing Review Planning Review Advisory Committee Wrap-up and Labeling Action

Guidance for Good Review Management Principles for PDUFA Products Application Receipt CDR assigns NDA/BLA no. EOP 2 Mtg. Pre-NDA Mtg. Presubmission Filing Fileabiliy Evaluation Encourage Regular Mtgs. Acknowledgement Letter Scientific & Reg. Advice Assignment of Review Team, Review Priority, Signature Authority Request Consults Inform of Filing Deficiencies Background Packages Submit App & User Fee Respond to Filing Deficiencies Davi, 2005

Guidance for Good Review Management Principles for PDUFA Products Labeling Negotiations Filing Final Division & Office Eval. & Action AC Mtg. Application Filed and Under Review Primary & Secondary Review IR/DR Letters Schedule AC AC Backgrounder, Questions, & Prep. Schedule AC AC Preparations Planned Amendments (safety update) Respond to IR/DR Letters Evaluate & Incorporate AC advice Labeling Negotiation Davi, 2005

The Shoe is on the Other Foot

Time for a Change? Is the GRMP another annoying regulatory/management burden? or “An OPPORTUNITY disguised as a PROBLEM*”? J. Michael

Industry/FDA Statisticians: Like an Old Married Couple?

Changes/Opportunities • • Can we change? What do we change? How do we change? Can we get better?

If I Ran the Zoo

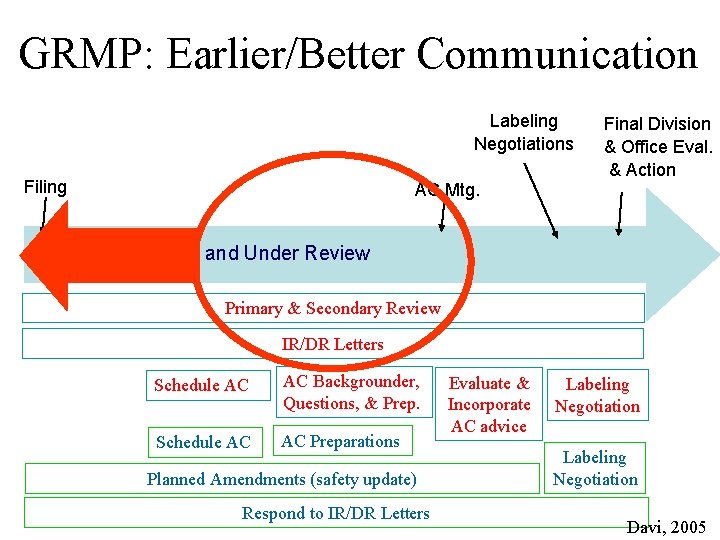
If I Ran the Zoo: Some of the Changes That I Would Like to See • Earlier/Better Communication: A Pre-NDA Statistics Meeting • Metadata Standards for Data and Analysis • More Guidance Relating to Statistics for Therapeutic Areas • Required Electronic Submission using the e. CTD (IND and NDA/CTD) • Technology-supported Tools for the IR/DR Process • “Adult Conversation” -- Sponsors submissions including reviews

GRMP: Earlier/Better Communication Labeling Negotiations Filing Final Division & Office Eval. & Action AC Mtg. Application Filed and Under Review Primary & Secondary Review IR/DR Letters Schedule AC AC Backgrounder, Questions, & Prep. Schedule AC AC Preparations Planned Amendments (safety update) Respond to IR/DR Letters Evaluate & Incorporate AC advice Labeling Negotiation Davi, 2005

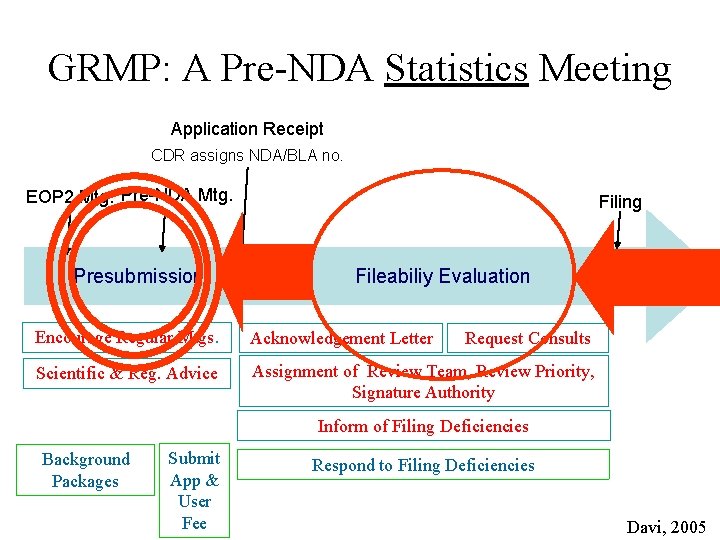
GRMP: A Pre-NDA Statistics Meeting Application Receipt CDR assigns NDA/BLA no. EOP 2 Mtg. Pre-NDA Mtg. Presubmission Filing Fileabiliy Evaluation Encourage Regular Mtgs. Acknowledgement Letter Scientific & Reg. Advice Assignment of Review Team, Review Priority, Signature Authority Request Consults Inform of Filing Deficiencies Background Packages Submit App & User Fee Respond to Filing Deficiencies Davi, 2005

Pre-NDA Statistics Meeting • Why not include in existing Pre-NDA meeting? – Wrong audience – (The bored and the lost) – No time – technical issues get moved to the end of the agenda – Timing - may be too early or too late • Will it add value? – Yes – it is necessary • How will it affect reviewers/sponsors processes and procedures?

Pre-NDA Statistics Topics • Review proposed primary analyses • Review proposed ADa. M and SDTM datasets • Discuss what additional analyses and ADa. M datasets might be useful (“Review Analysis Files”) • Discuss submission of programs – Analysis dataset creation programs – Analysis generation programs – Timing and functionality of submission of programs • Evaluate “test data” – “pre-e-Submission”

Pre-e-Submission Data and Metadata • Provides valuable feedback to sponsor • Allows agency to test compatibility with standard tools and databases • Tests content and structure of metadata, documentation and programs • Identifies technical problems early • Highlights possible areas of misunderstanding and differences in interpretation • “Tunes” in the reviewer

How to Provide “Clear Communication” • Use the e. CTD to provide a common organization and structure to the document. – Makes components easy to find – Allows standard tools to be used for navigation, browsing • Use ADa. M and SDTM dataset standards and metadata to describe the submitted data – Common look and feel for data, both within and across submissions – Allows standard tools to be used for navigation, browsing and analysis

Metadata Standards for Data and Analysis • Enable reviewers to understand, replicate, explore, confirm, reuse, etc. • Clear, unambiguous communication of decisions, analysis and results • Underlying principles: – Can a reviewing statistician understand? – Can a reviewing statistician efficiently: • Quality Assure? • Validate? • Analyze?

Metadata Standards for Data and Analysis 1. 2. 3. 4. Analysis Datasets Analysis Variables Programs

More Statistical Guidance: General Principles & Therapy-Specific

GRMP: Motivates All of Us to Wear the Same Better Faster Shoes