Preparing Solutions Terms to Know Standard Solution A










- Slides: 16

Preparing Solutions

Terms to Know • Standard Solution • A solution that has a known concentration • Stock Solution • A solution that is in stock or on the shelf already prepared, usually a concentrated solution. • Pure Water • Deionized or distilled water

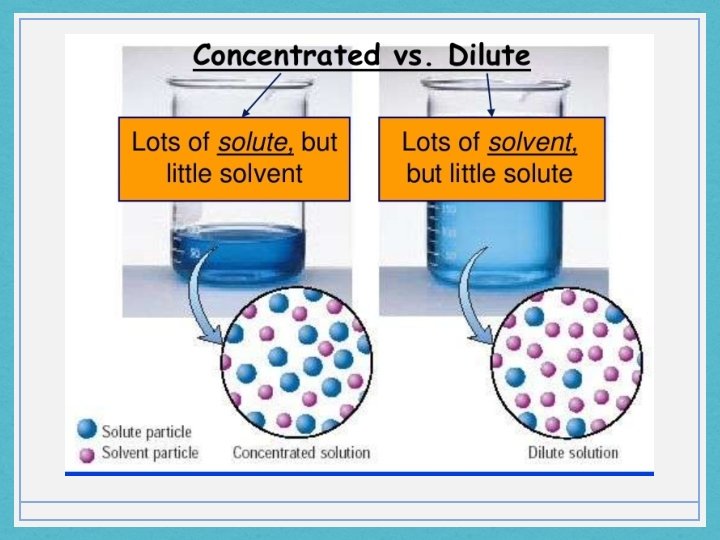
• Solute • The substance that is dissolved in a solution • Solvent • The substance in which a solute dissolves • Dilution • The process of decreasing the concentration of a solution, usually by adding more solvent. There are two ways to prepare a standard solution

A. Preparing a Standard Solution from a Solid - Using a Volumetric Flask • Precision equipment is required to measure the mass of solute and volume of solution. • To make a solution with known concentration, you can dissolve a solute of known mass in a special vessel called a volumetric flask.

• A volumetric flask is a glass container that measures a very accurate and precise volume.

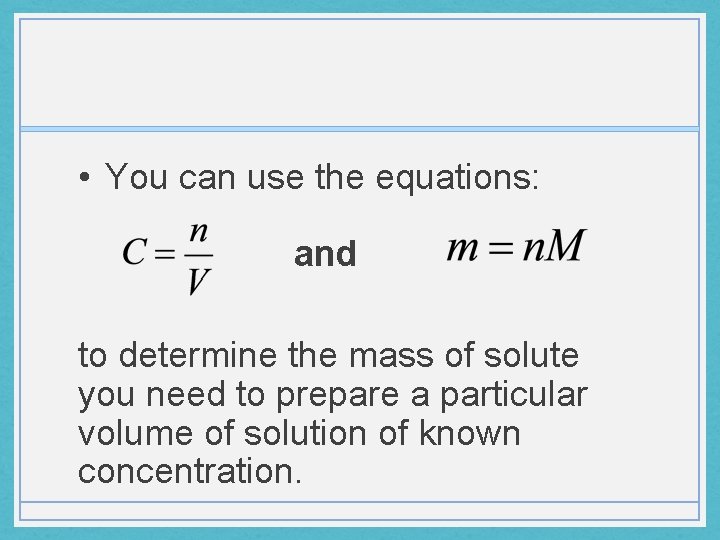
• You can use the equations: and to determine the mass of solute you need to prepare a particular volume of solution of known concentration.

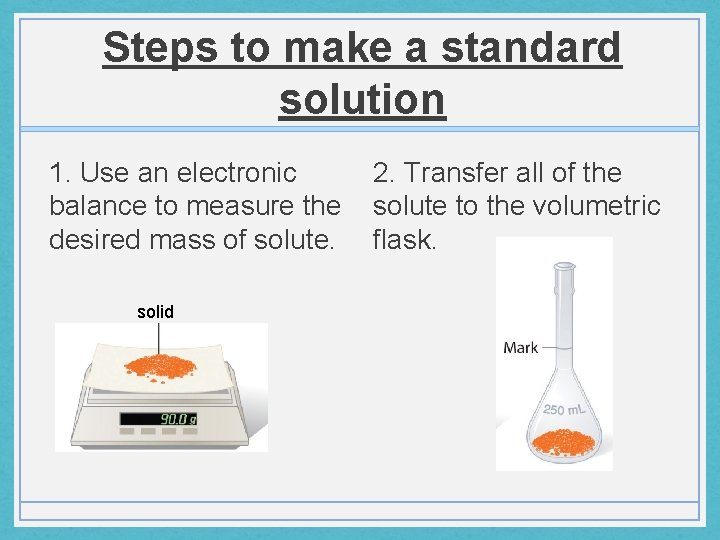
Steps to make a standard solution 1. Use an electronic 2. Transfer all of the balance to measure the solute to the volumetric desired mass of solute. flask. solid

3. Add water (the solvent) to the volumetric flask until it is about half full. Then swirl the flask to dissolve the solid in the water completely.

4. Fill the flask by adding water slowly and carefully, using a wash bottle, until the bottom of the meniscus just touches the etched line the flask. 5. Stopper the flask and mix the contents by repeatedly inverting the flask. Inverting means that you flip the flask upside-down and the right side up.

Example #1: A solution of potassium permanganate in water, KMn. O 4(aq), can be used as an antiseptic and a fungicide. How would you make 250. 0 m. L of 0. 100 mol/L KMn. O 4(aq), using a 250. 0 m. L volumetric flask, water, an electronic balance, and solid crystals of potassium permanganate?

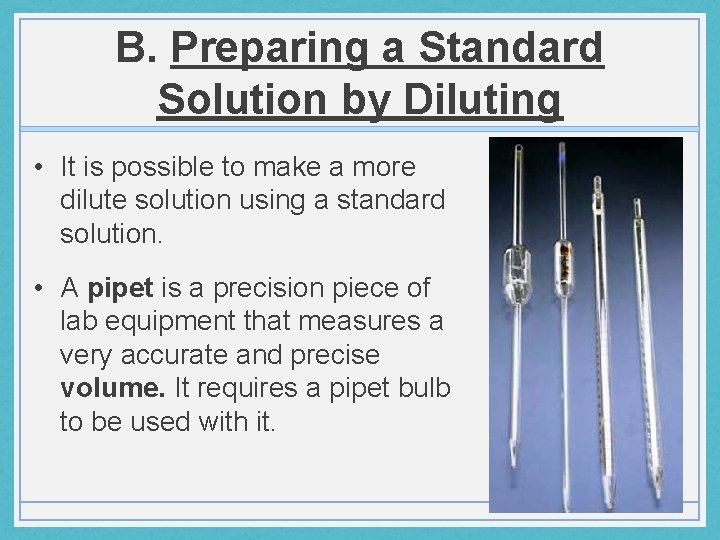
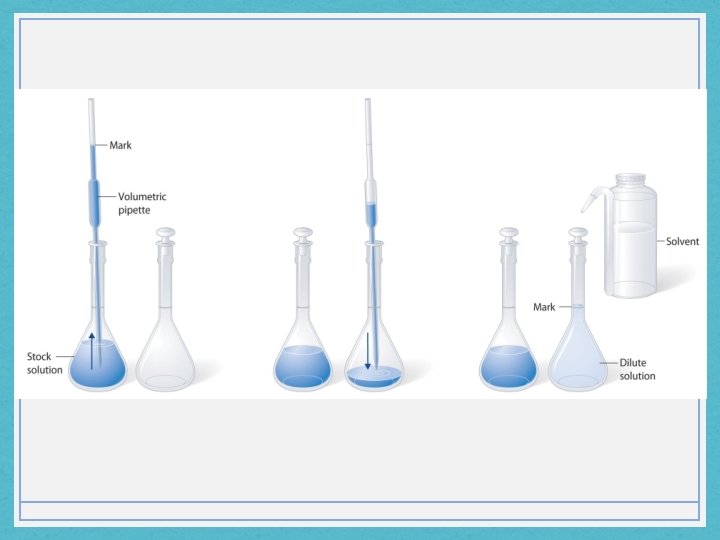
B. Preparing a Standard Solution by Diluting • It is possible to make a more dilute solution using a standard solution. • A pipet is a precision piece of lab equipment that measures a very accurate and precise volume. It requires a pipet bulb to be used with it.


Steps to make a diluted solution from a stock solution 1. Determine the amount of standard solution required, and use the pipet to measure it out. Put this volume into a volumetric flask. 2. Add the rest of the solvent to the flask until the meniscus appears to touch the line found around the neck of the flask. 3. Add the stopper and mix the contents by repeatedly inverting the flask. Before and after dilution, the number of molecules and moles do not change. (of the solute)


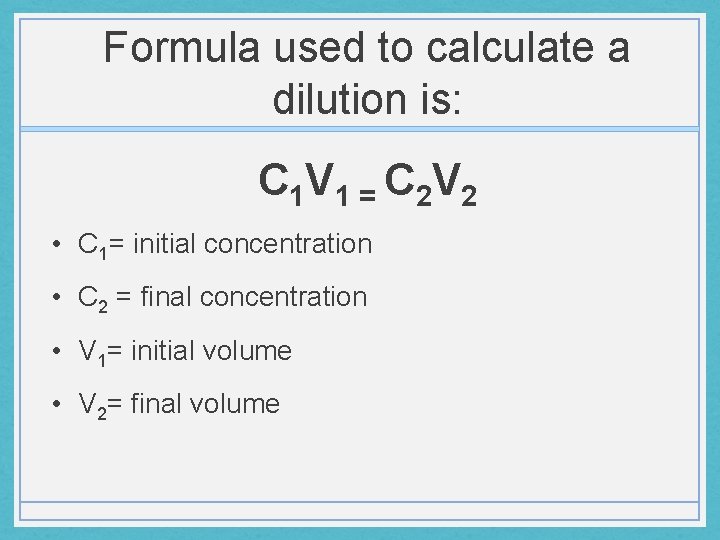
Formula used to calculate a dilution is: C 1 V 1 = C 2 V 2 • C 1= initial concentration • C 2 = final concentration • V 1= initial volume • V 2= final volume

Example #2: For an experiment, it is necessary to make 2. 0 L of a 0. 10 mol/L sulfuric acid solution. This acid is usually sold as an 18 mol/L concentrated solution. How much of the concentrated solution should be used to make the new solution?