PREPARING SOLUTIONS AND REAGENTS II SOLUTIONS WITH MORE













- Slides: 18

PREPARING SOLUTIONS AND REAGENTS II

SOLUTIONS WITH MORE THAN ONE SOLUTE RECIPE I Na 2 HPO 4 KH 2 PO 4 0. 4% glycerol 6 g 3 g 10 m. L Dissolve in water. Bring to a volume of 1 liter. Recipe lists amounts of solutes.

RECIPE II 1 M Mg. Cl 2 0. 1 M Tris Gives the final concentration of each solute. You must calculate the amount of each to use. lseidman@matcmadison. edu

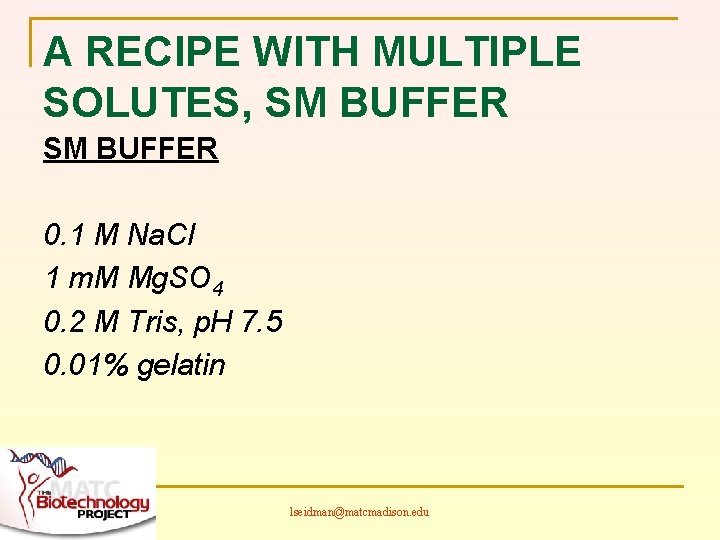
A RECIPE WITH MULTIPLE SOLUTES, SM BUFFER 0. 1 M Na. Cl 1 m. M Mg. SO 4 0. 2 M Tris, p. H 7. 5 0. 01% gelatin lseidman@matcmadison. edu

STRATEGY 1: PREPARING SM BUFFER WITHOUT STOCK SOLUTIONS, OVERVIEW: n Prepare a solution of 0. 2 M Tris, p. H 7. 5. n Calculate grams of each of the other solutes required. n Weigh out solutes and dissolve directly in Tris buffer. lseidman@matcmadison. edu

STRATEGY 1 1. Decide how much buffer to make, for example, 1 liter. 2. 1 liter of 0. 2 M Tris requires 24. 2 g of Tris base. (MW = 121. 1 g/mole. ) 3. Dissolve Tris in about 700 m. L of water and bring p. H to 7. 5. Do not bring Tris to volume. lseidman@matcmadison. edu

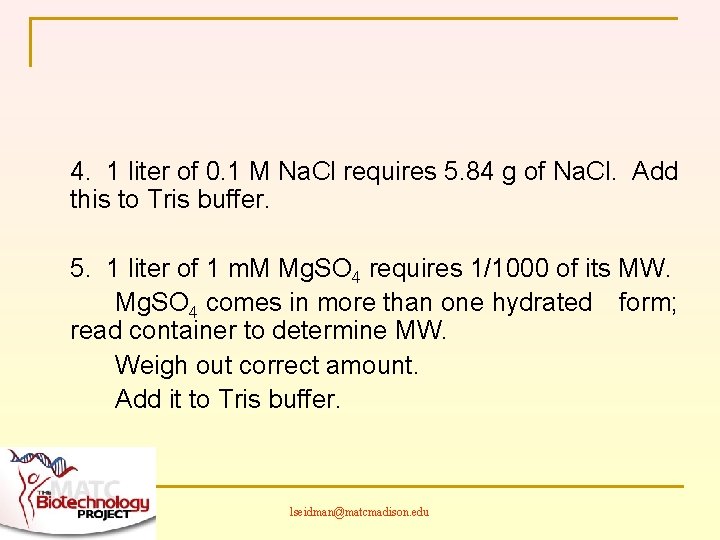
4. 1 liter of 0. 1 M Na. Cl requires 5. 84 g of Na. Cl. Add this to Tris buffer. 5. 1 liter of 1 m. M Mg. SO 4 requires 1/1000 of its MW. Mg. SO 4 comes in more than one hydrated form; read container to determine MW. Weigh out correct amount. Add it to Tris buffer. lseidman@matcmadison. edu

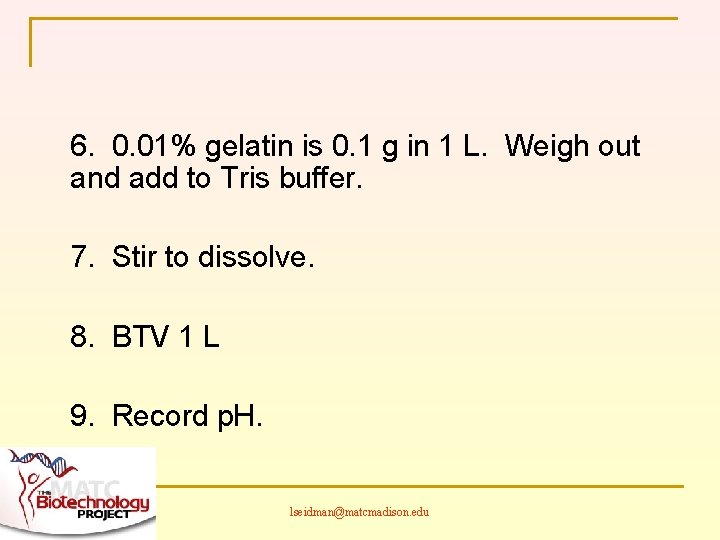
6. 0. 01% gelatin is 0. 1 g in 1 L. Weigh out and add to Tris buffer. 7. Stir to dissolve. 8. BTV 1 L 9. Record p. H. lseidman@matcmadison. edu

STRATEGY 2: PREPARING SM BUFFER WITH STOCK SOLUTIONS n In overview: n Four solutes are each prepared separately as concentrated stock solutions. n . lseidman@matcmadison. edu

n When the four stocks are combined, they dilute one another to the proper final concentrations lseidman@matcmadison. edu

STEPS ARE: 1. Prepare a stock solution of Tris buffer at p. H 7. 5. a. No set rule as to what should be concentration of stock. b. To make 1 L of 1 M stock, dissolve 121. 1 g of Tris base in about 900 m. L of water. c. Bring p. H to 7. 5. d. BTV 1 L.

2. Prepare a stock solution of magnesium sulfate, for example, 1 M. q To make 100 m. L, dissolve 0. 1 MW of Mg. SO 4 in water, bring to a volume of 100 m. L. lseidman@matcmadison. edu

Prepare a stock solution of Na. Cl, for example, 1 M. q To make 100 m. L of stock, dissolve 5. 84 grams in water and BTV 100 m. L. lseidman@matcmadison. edu

Prepare a stock solution of gelatin, for example, 1%. 4. q Dissolve 1 g in a final volume of 100 m. L of water. lseidman@matcmadison. edu

5. To make the final solution, combine the right amounts of each stock. q q Since this is a situation where stocks are diluted, use the C 1 V 1 = C 2 V 2 equation Four times, once for each solute. lseidman@matcmadison. edu

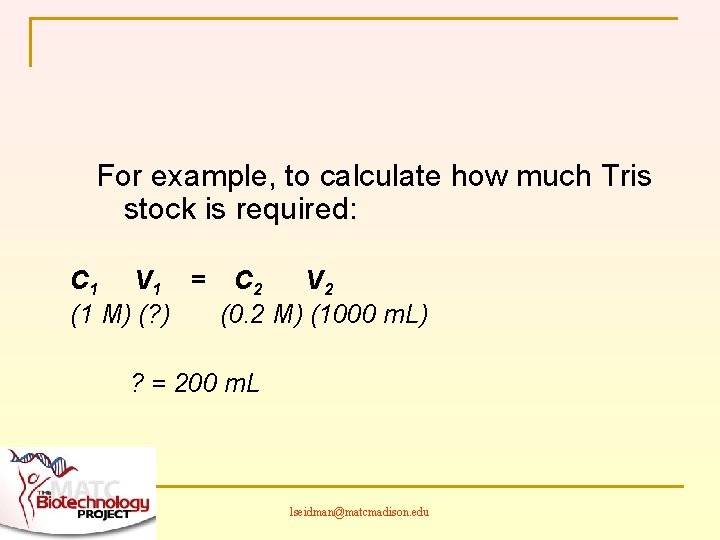
For example, to calculate how much Tris stock is required: C 1 V 1 = C 2 V 2 (1 M) (? ) (0. 2 M) (1000 m. L) ? = 200 m. L lseidman@matcmadison. edu

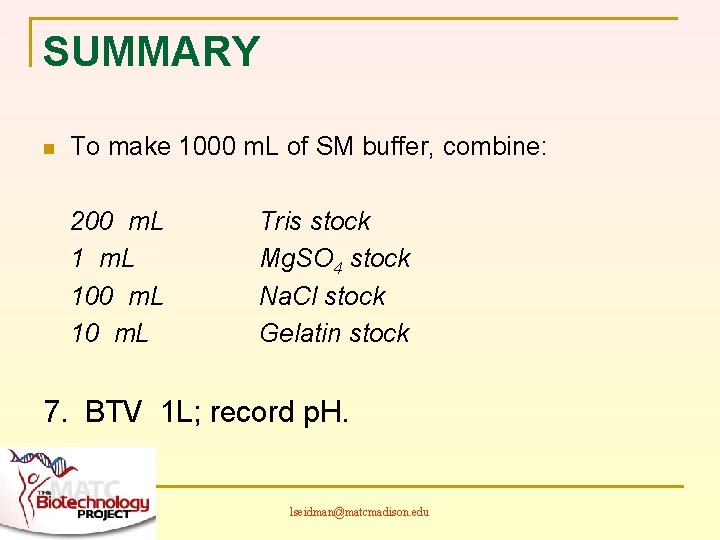
SUMMARY n To make 1000 m. L of SM buffer, combine: 200 m. L 100 m. L 10 m. L Tris stock Mg. SO 4 stock Na. Cl stock Gelatin stock 7. BTV 1 L; record p. H. lseidman@matcmadison. edu

WHICH STRATEGY? n Both strategy 1 and 2 are correct. n Generally efficient to make stock solutions of frequently used solutes because weighing out chemicals is time-consuming. lseidman@matcmadison. edu