PREPARING LABORATORY SOLUTIONS AND REAGENTS Important Practices Use

PREPARING LABORATORY SOLUTIONS AND REAGENTS

Important Practices: • • Use distilled or deionized water to make solutions Prepare solutions in clean containers
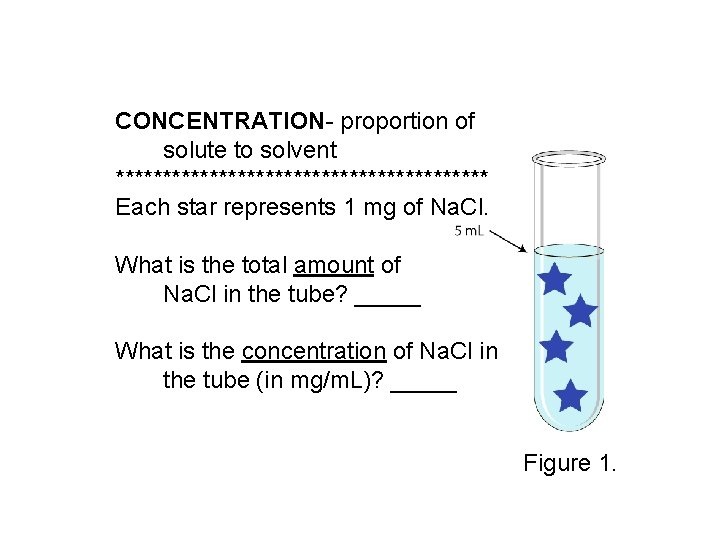
CONCENTRATION- proportion of solute to solvent ******************** Each star represents 1 mg of Na. Cl. What is the total amount of Na. Cl in the tube? _____ What is the concentration of Na. Cl in the tube (in mg/m. L)? _____ Figure 1.

Concentration is measured in several ways in biotech labs • MASS PER VOLUME • • MOLARITY PERCENTS (Three kinds)
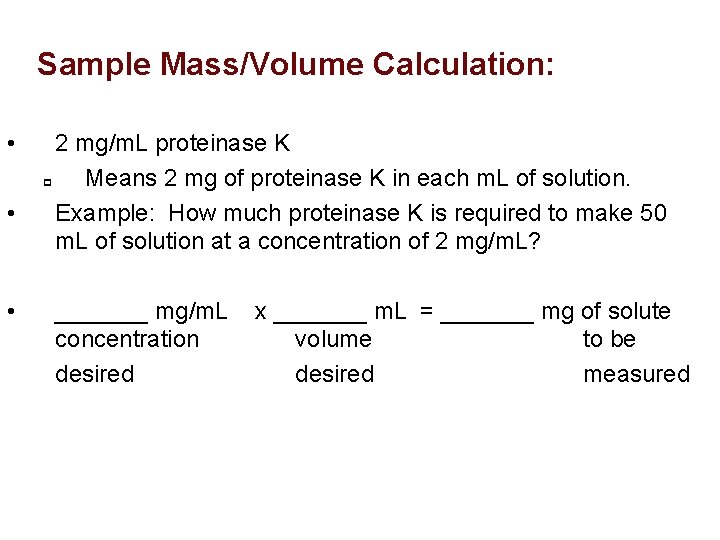
Sample Mass/Volume Calculation: • • • 2 mg/m. L proteinase K p Means 2 mg of proteinase K in each m. L of solution. Example: How much proteinase K is required to make 50 m. L of solution at a concentration of 2 mg/m. L? _______ mg/m. L concentration desired x _______ m. L = _______ mg of solute volume to be desired measured

Preparing Dilute Solutions From Concentrated Ones • • Concentrated solution = stock solution Use this equation to decide how much stock solution you will need: C 1 V 1=C 2 V 2 C 1 = concentration of stock solution C 2 = concentration you want your dilute solution to be V 1 = how much stock solution you will need V 2 = how much of the dilute solution you want to make

“X” Solutions • • The concentration of a stock solution is sometimes written with an “X”. The “X” is how many more times the stock is than normal.

Sample Dilution Calculation: • A can of frozen orange juice is labeled 4 X. How would you dilute it to make 1 L of drinkable juice?
- Slides: 8