PREPARING FOR PREP UNDERSTANDING ACCESS AND CLINICAL PRACTICE


































- Slides: 56

PREPARING FOR PREP: UNDERSTANDING ACCESS AND CLINICAL PRACTICE Janie Caplan, MD, Infectious Diseases Fellow, UCLA Gifty-Maria Ntim, MD, MPH Medical Director, APLA Health & Wellness David Evans, Director of Research Advocacy, Project Inform

Describe Pr. EP Usage and Approved Medications Currently Provided at Community Health Centers and their Effectiveness Gifty-Maria Ntim, MD, MPH Medical Director, APLA Health & Wellness

Disclosures I have nothing to disclose personally however APLA Health & Wellness has received funding in the past from Gilead

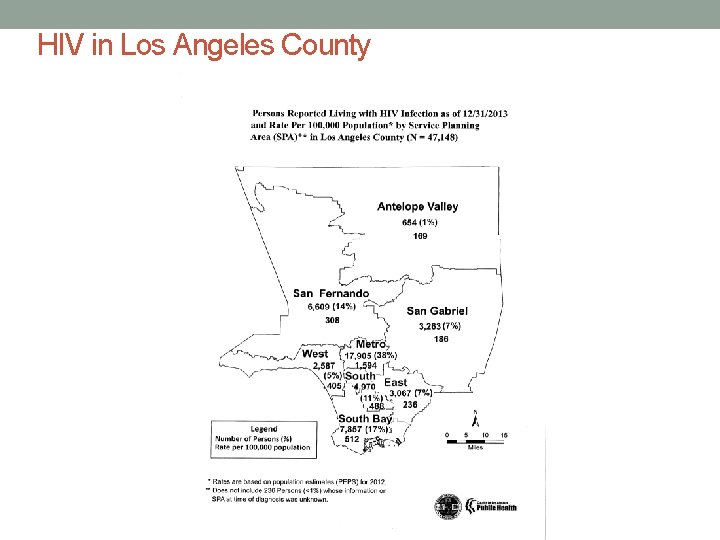
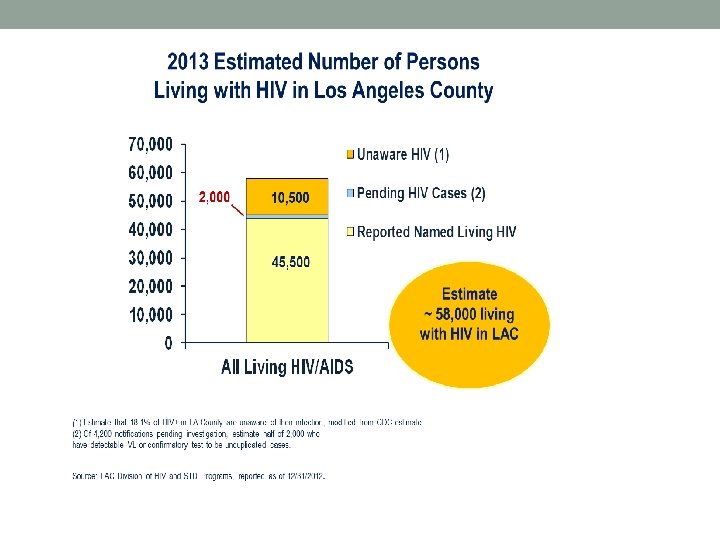
HIV in Los Angeles County


HIV Prevention Comprehensive Approach • Behavioral Interventions • Biomedical Interventions • Tas. P • Pr. EP • PEP • Structural Interventions

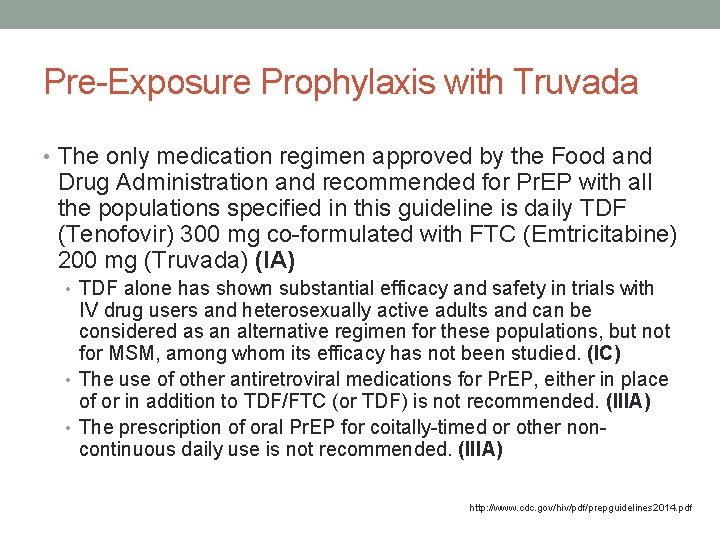
Pre-Exposure Prophylaxis with Truvada • The only medication regimen approved by the Food and Drug Administration and recommended for Pr. EP with all the populations specified in this guideline is daily TDF (Tenofovir) 300 mg co-formulated with FTC (Emtricitabine) 200 mg (Truvada) (IA) • TDF alone has shown substantial efficacy and safety in trials with IV drug users and heterosexually active adults and can be considered as an alternative regimen for these populations, but not for MSM, among whom its efficacy has not been studied. (IC) • The use of other antiretroviral medications for Pr. EP, either in place of or in addition to TDF/FTC (or TDF) is not recommended. (IIIA) • The prescription of oral Pr. EP for coitally-timed or other noncontinuous daily use is not recommended. (IIIA) http: //www. cdc. gov/hiv/pdf/prepguidelines 2014. pdf

Let’s Talk About Sex

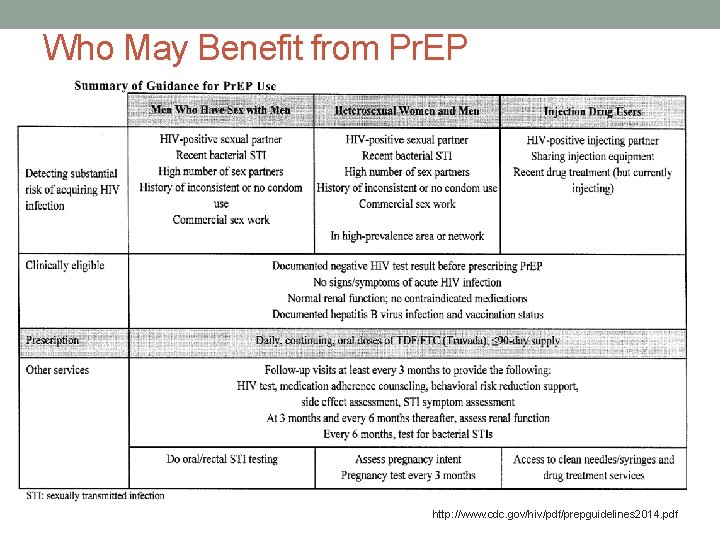
Who May Benefit from Pr. EP http: //www. cdc. gov/hiv/pdf/prepguidelines 2014. pdf

California Pr. EP Coverage at a Glance • Medi-Cal covers Pr. EP 100% if a single household income is less than $16, 243 a year • Covered California offers reasonable access if you choose an appropriate plan (Bronze, Silver, Enhanced Silver 73, 87, 94, Gold, Platinum) • Gilead offers co-pay assistance to help cover up to $3, 600 /year Assistance can be used to cover pharmacy deductibles and co-pays for medication itself (no income requirements) • Gilead offers free medication to those who do not have insurance (i. e. undocumented individuals, those who are not eligible to sign-up through market place , Medi-Cal pending, waiting for open enrollment, etc)

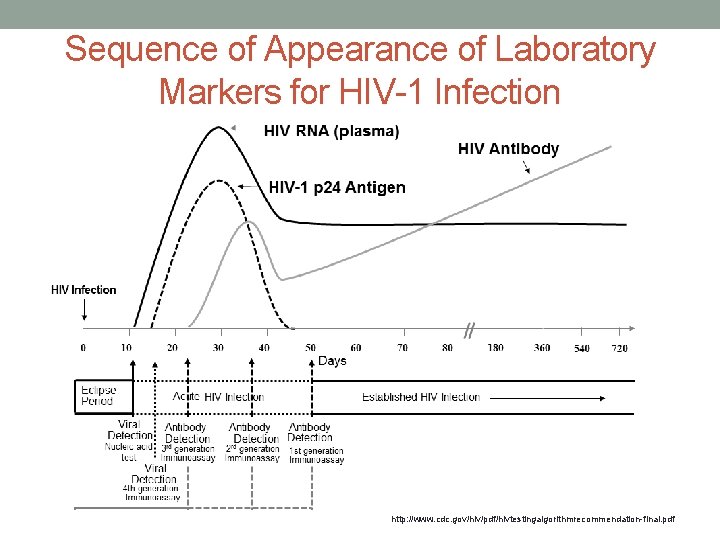
Sequence of Appearance of Laboratory Markers for HIV-1 Infection http: //www. cdc. gov/hiv/pdf/hivtestingalgorithmrecommendation-final. pdf

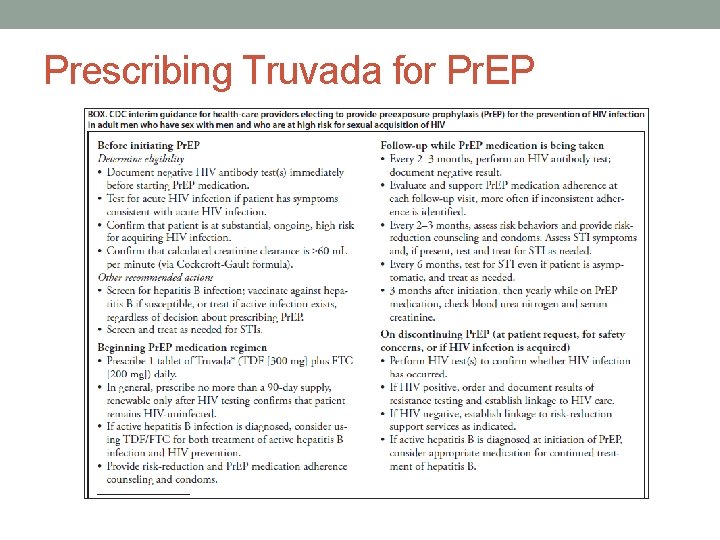
Prescribing Truvada for Pr. EP

Adherence and Counseling Establish trust and bidirectional communication with your patient • Provide simple explanations and education • Discuss medication dosage and schedule • Discuss management of common side effects • Discuss the relationship of adherence to the efficacy of Pr. EP • Discuss signs and symptoms of acute HIV infection and recommended actions Support adherence • Tailor daily dose to patient’s daily routine • Identify reminders and devices to minimize forgetting doses • Identify and address barriers to adherence Monitor medication adherence in a non-judgmental manner • Normalize occasional missed doses, while ensuring patient understands importance of daily dosing for optimal protection e. g. ‘how many doses did you miss in the last 7 days? ’ • Reinforce success • Identify factors interfering with adherence and plan with patient to address them • Assess side effects and plan how to manage them http: //www. cdc. gov/hiv/pdf/prepguidelines 2014. pdf

Concerns Raised by Pr. EP • Side-effects and toxicity • Drug resistance • Adherence • Risk compensation • Access • Cost • Truvada’s ‘street value’

Common Asked Questions 1. 2. 3. 4. 5. 6. 7. 8. How soon after starting Pr. EP can I have some protection? What side effects should I watch for? What happens when I miss a dose of Truvada? Why do I need to come in for refills? Do I still need to use condoms? What about the guy who was taking Pr. EP who recently tested positive for HIV? Is my body going to look weird after I start taking Truvada? Will Truvada affect my sex drive?

Sexually Transmitted Infections and Pr. EP • Test for STIs every 3 m and as needed based on exposure vs. testing every 6 m per CDC guidelines

Helpful ICD 10 Codes • Z 72. 51 High Risk Heterosexual Behavior • Z 72. 52 High Risk Homosexual Behavior • Z 72. 53 High Risk Bisexual Behavior

Margaret “Janie” Caplan, MD Fellow physician Division of Infectious Diseases David Geffen School of Medicine at UCLA EVIDENCE FOR USE OF PRE-EXPOSURE PROPHYLAXIS IN AT RISK POPULATIONS

Disclosures § I have nothing to disclose!

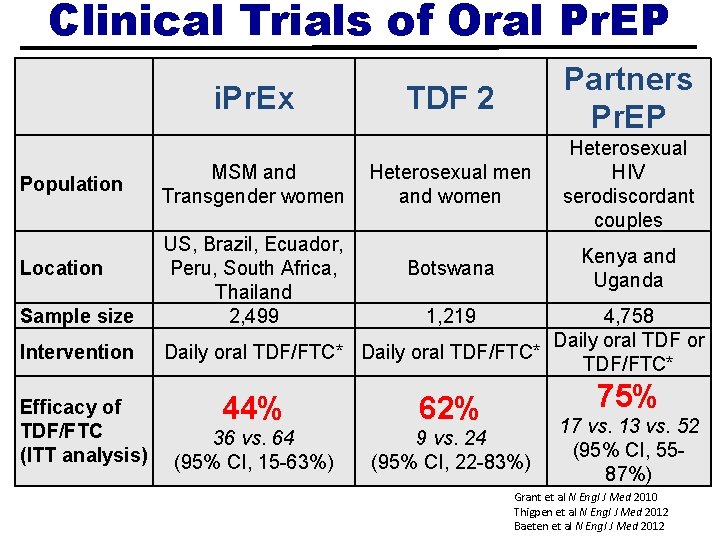
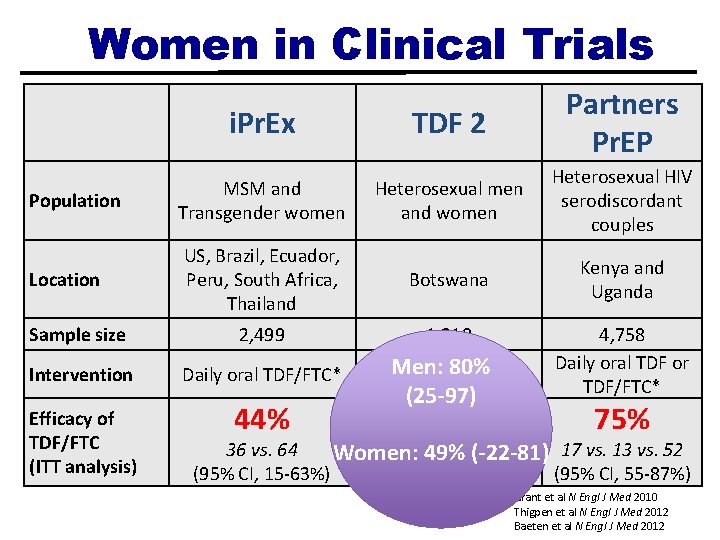
Clinical Trials of Oral Pr. EP i. Pr. Ex Population Location Sample size Intervention Efficacy of TDF/FTC (ITT analysis) MSM and Transgender women US, Brazil, Ecuador, Peru, South Africa, Thailand 2, 499 TDF 2 Partners Pr. EP Heterosexual men and women Heterosexual HIV serodiscordant couples Botswana Kenya and Uganda 1, 219 4, 758 Daily oral TDF or Daily oral TDF/FTC* 75% 44% 62% 36 vs. 64 (95% CI, 15 -63%) 9 vs. 24 (95% CI, 22 -83%) 17 vs. 13 vs. 52 (95% CI, 5587%) Grant et al N Engl J Med 2010 Thigpen et al N Engl J Med 2012 Baeten et al N Engl J Med 2012

*All as part of a comprehensive prevention package* § HIV testing § Risk-reduction counseling and condoms § Diagnosis and treatment of symptomatic STIs (gonorrhea, chlamydia, syphilis, and HSV-2), as well as screening in asymptomatic individuals and partners § +/- Referral for PEP if reporting a recent exposure to HIV § +/- Hepatitis B vaccination

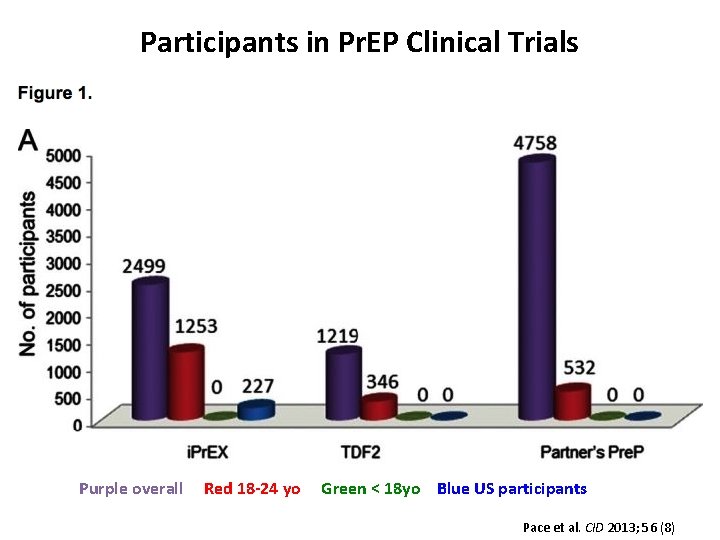
Participants in Pr. EP Clinical Trials Purple overall Red 18 -24 yo Green < 18 yo Blue US participants Pace et al. CID 2013; 56 (8)

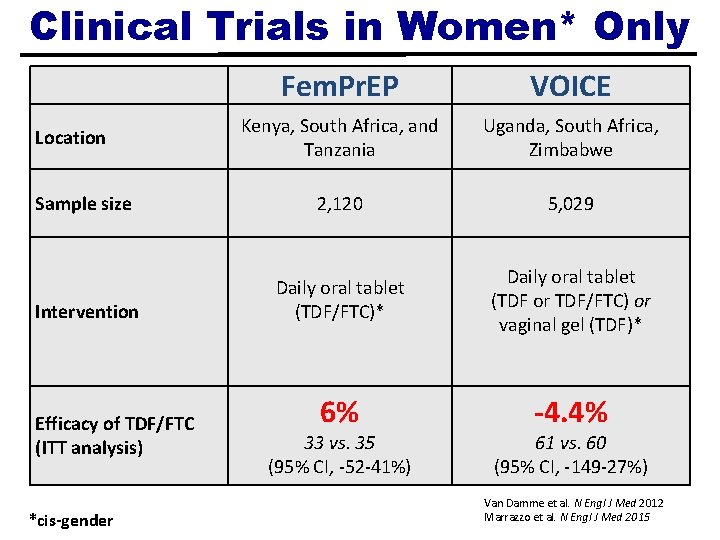
Clinical Trials in Women* Only Fem. Pr. EP VOICE Kenya, South Africa, and Tanzania Uganda, South Africa, Zimbabwe Sample size 2, 120 5, 029 Intervention Daily oral tablet (TDF/FTC)* Daily oral tablet (TDF or TDF/FTC) or vaginal gel (TDF)* 6% -4. 4% Location Efficacy of TDF/FTC (ITT analysis) *cis-gender 33 vs. 35 (95% CI, -52 -41%) 61 vs. 60 (95% CI, -149 -27%) Van Damme et al. N Engl J Med 2012 Marrazzo et al. N Engl J Med 2015

Women in Clinical Trials i. Pr. Ex TDF 2 Partners Pr. EP Population MSM and Transgender women Heterosexual men and women Heterosexual HIV serodiscordant couples Location US, Brazil, Ecuador, Peru, South Africa, Thailand Botswana Kenya and Uganda Sample size 2, 499 1, 219 Intervention Daily oral TDF/FTC* Men: Daily oral 80% TDF/FTC* 4, 758 Daily oral TDF or TDF/FTC* Efficacy of TDF/FTC (ITT analysis) 44% 62% 75% (25 -97) 36 vs. 64 vs. 24(-22 -81) 17 vs. 13 vs. 52 Women: 949% (95% CI, 15 -63%) (95% CI, 22 -83%) (95% CI, 55 -87%) Grant et al N Engl J Med 2010 Thigpen et al N Engl J Med 2012 Baeten et al N Engl J Med 2012

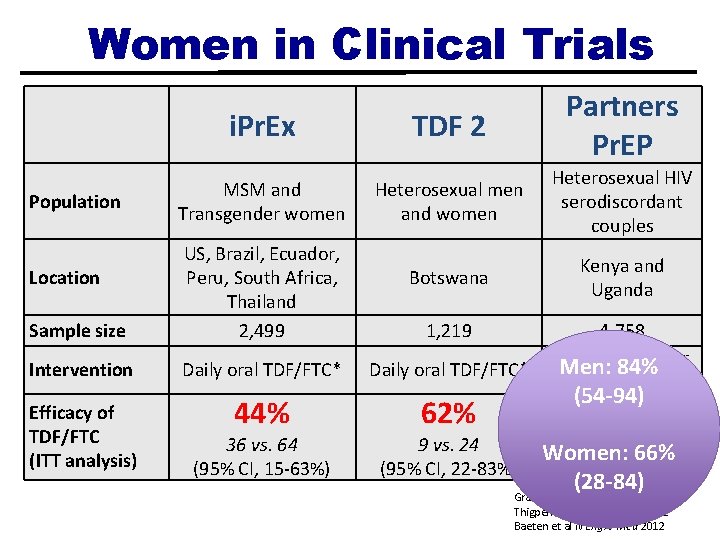
Women in Clinical Trials Population i. Pr. Ex TDF 2 Partners Pr. EP MSM and Transgender women Heterosexual men and women Heterosexual HIV serodiscordant couples Botswana Kenya and Uganda Sample size US, Brazil, Ecuador, Peru, South Africa, Thailand 2, 499 Intervention Daily oral TDF/FTC* Efficacy of TDF/FTC (ITT analysis) 44% 62% Location 36 vs. 64 (95% CI, 15 -63%) 1, 219 9 vs. 24 (95% CI, 22 -83%) 4, 758 Daily TDF or Men: oral 84% TDF/FTC* (54 -94) 75% 17 vs. 13 66% vs. 52 Women: (95% CI, 55 -87%) (28 -84) Grant et al N Engl J Med 2010 Thigpen et al N Engl J Med 2012 Baeten et al N Engl J Med 2012

What happened? ADHERENCE Clinical Efficacy Pharmacology

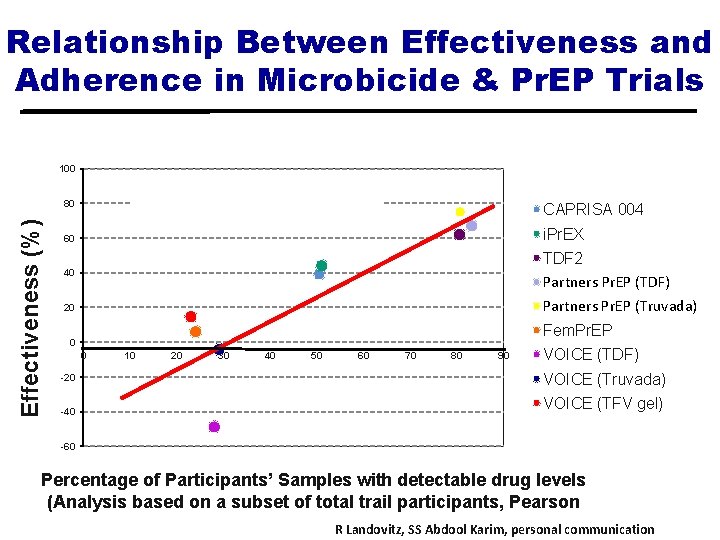
Relationship Between Effectiveness and Adherence in Microbicide & Pr. EP Trials 100 Pearson correlation = 0. 86, p=0. 003 Effectiveness (%) 80 CAPRISA 004 60 i. Pr. EX 40 TDF 2 Partners Pr. EP (TDF) Partners. Prep (TDF) 20 Partners Pr. EP (Truvada) Partners. Pre. P (FTC) Fem. Pr. EP 0 0 -20 -40 10 20 30 40 50 60 70 80 90 VOICE (TDF) VOICE (Truvada) VOICE (TFV gel) -60 Percentage of Participants’ Samples with detectable drug levels (Analysis based on a subset of total trail participants, Pearson correlation = 0. 86, p=0. 003) R Landovitz, SS Abdool Karim, personal communication

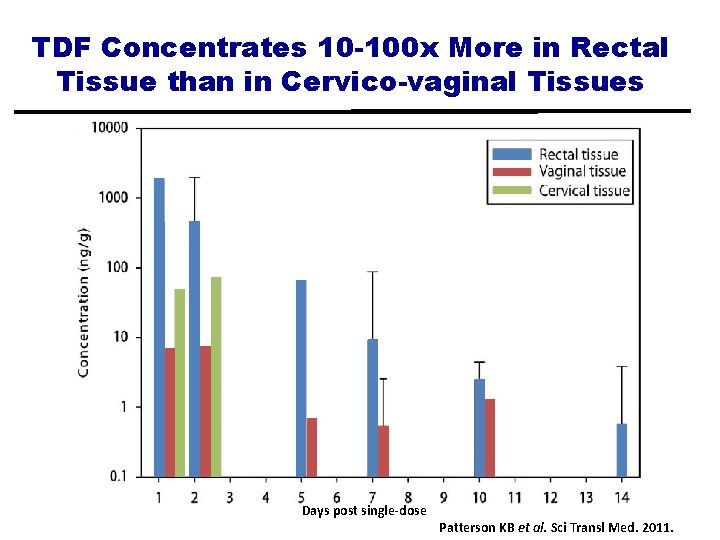
TDF Concentrates 10 -100 x More in Rectal Tissue than in Cervico-vaginal Tissues Days post single-dose Patterson KB et al. Sci Transl Med. 2011.

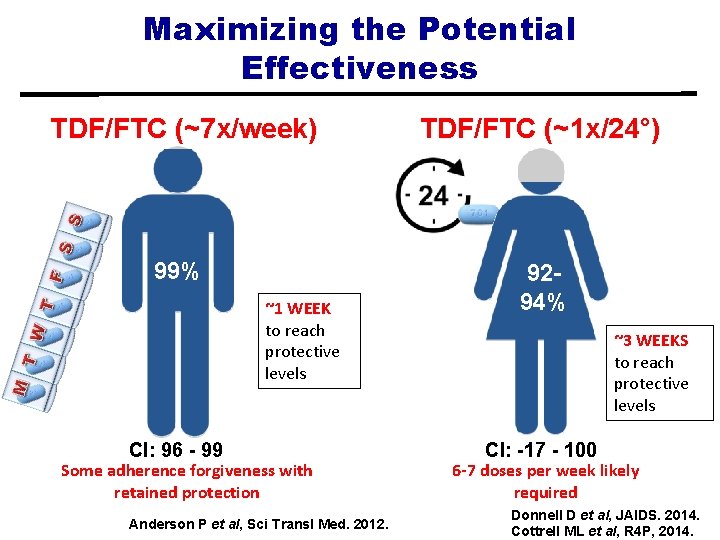
Maximizing the Potential Effectiveness TDF/FTC (~7 x/week) TDF/FTC (~1 x/24°) 99% 9294% ~1 WEEK to reach protective levels CI: 96 - 99 Some adherence forgiveness with retained protection Anderson P et al, Sci Transl Med. 2012. ~3 WEEKS to reach protective levels CI: -17 - 100 6 -7 doses per week likely required Donnell D et al, JAIDS. 2014. Cottrell ML et al, R 4 P, 2014.

Dosing Strategies § Intermittent dosing is NOT recommended at this point. § IPERGAY study: “On Demand” Pr. EP with TDF/FTC, only in MSM 1 § HPTN 067 (ADAPT): non-daily Pr. EP in South African women 2 § Prelim data: Daily dosing fostered better adherence, better coverage of potential sexual exposure, and more sustained use § Take Home Point: Daily dosing! 1. 2. Molina JM, et al. CROI, 2015. Bekker LG, et al. CROI, 2015.

Efficacy Depends on Adherence § § Next Step Counseling 1 CDC Guidance 2 Text messaging 3, 4 “Smart” devices 5, 6 1. Amico KR et al. AIDS Behav. 2012. 2. CDC Clinical Practice Guidelines. 2014. 3. Finitsis DJ et al. PLo. S ONE. 2014. 4. Moore D et al. CCTG 595. 5. Bekker LG et al. HPTN 067 6. Gulick RM et al. HPTN 069

Risk Compensation § Theory that people adjust their behaviors in response to perceived level of risk § Historical example: Birth control and concern that its increased availability would promote risky sexual behavior § Does Pr. EP use result in increase in risky behavior (e. g. less condom use)? § Pr. EP trials have not seen risk compensation. § HOWEVER, in these trials, participants knew they might be getting a placebo. § What will happen in the real world setting? § i. Pr. EX Open Label Extension (OLE): no significant change in sexual practices

Sexually Transmitted Infections § High incidence of STIs during follow-up in Pr. EP studies: increase in unprotected sex or increase in detection/screening? § Screening for STIs during Pr. EP use § CDC recommendation: Based on symptoms, and/or every 6 months for bacterial STIs § Evidence from Pr. EP implementation studies in New York and San Francisco suggesting more frequent, routine screening q 3 months might detect more incident STIs

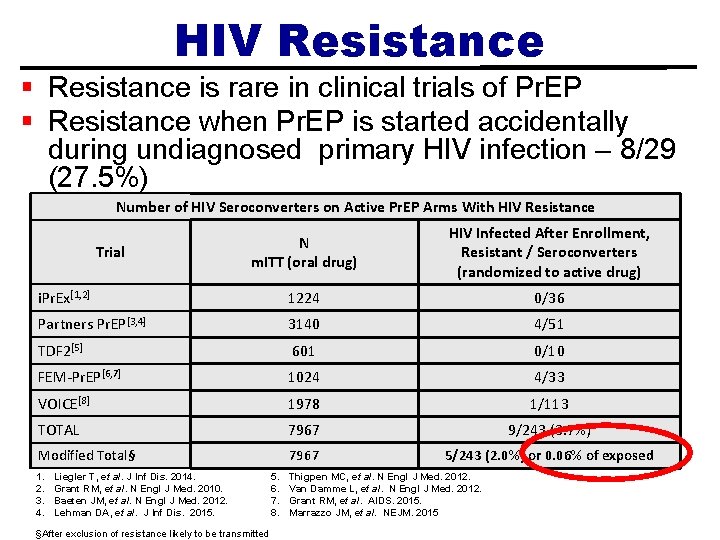
HIV Resistance § Resistance is rare in clinical trials of Pr. EP § Resistance when Pr. EP is started accidentally during undiagnosed primary HIV infection – 8/29 (27. 5%) Number of HIV Seroconverters on Active Pr. EP Arms With HIV Resistance N m. ITT (oral drug) HIV Infected After Enrollment, Resistant / Seroconverters (randomized to active drug) i. Pr. Ex[1, 2] 1224 0/36 Partners Pr. EP[3, 4] 3140 4/51 TDF 2[5] 601 0/10 FEM-Pr. EP[6, 7] 1024 4/33 VOICE[8] 1978 1/113 TOTAL 7967 9/243 (3. 7%) Modified Total§ 7967 5/243 (2. 0%) or 0. 06% of exposed Trial 1. 2. 3. 4. Liegler T, et al. J Inf Dis. 2014. Grant RM, et al. N Engl J Med. 2010. Baeten JM, et al. N Engl J Med. 2012. Lehman DA, et al. J Inf Dis. 2015. §After exclusion of resistance likely to be transmitted 5. 6. 7. 8. Thigpen MC, et al. N Engl J Med. 2012. Van Damme L, et al. N Engl J Med. 2012. Grant RM, et al. AIDS. 2015. Marrazzo JM, et al. NEJM. 2015

Ongoing Research § Other oral antiretrovirals as Pr. EP § Maraviroc (HPTN 069) § Long Acting Therapies § Vaginal rings – dapivirine § Injectables – rilpivirine; cabotegravir § Immunotherapies – VRC 01 § Intermittent (i)Pr. EP § Demonstration projects § Cis-gender and transgender women

22 February 2016

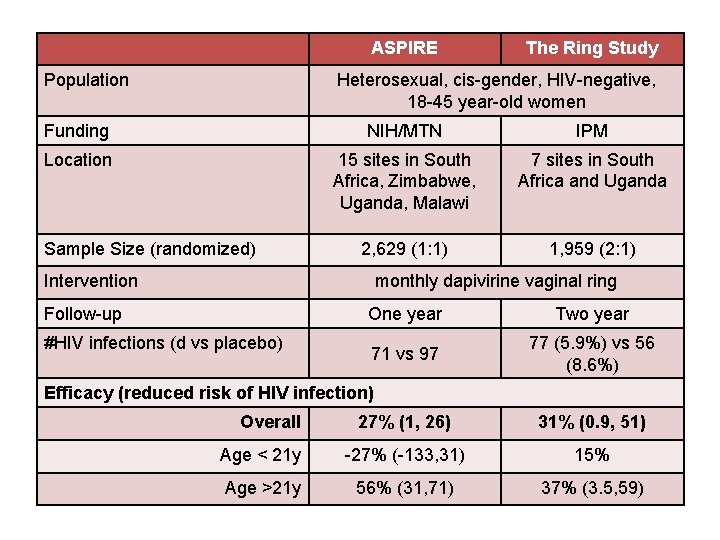
ASPIRE Population The Ring Study Heterosexual, cis-gender, HIV-negative, 18 -45 year-old women Funding NIH/MTN IPM Location 15 sites in South Africa, Zimbabwe, Uganda, Malawi 7 sites in South Africa and Uganda 2, 629 (1: 1) 1, 959 (2: 1) Sample Size (randomized) Intervention monthly dapivirine vaginal ring Follow-up #HIV infections (d vs placebo) One year Two year 71 vs 97 77 (5. 9%) vs 56 (8. 6%) Efficacy (reduced risk of HIV infection) Overall 27% (1, 26) 31% (0. 9, 51) Age < 21 y -27% (-133, 31) 15% Age >21 y 56% (31, 71) 37% (3. 5, 59)

Ongoing Research § Local demonstration projects funded by California HIV/AIDS Research Program (CHRP) § Currently completing follow-up of MSM projects § Project in cis-gender women nearing start of enrollment § Funding for projects focusing on transgender persons

Ongoing Research AEGi. S – Pr. EP Adherence Enhancement Guided by i. TAB and Drug Levels for Women § Los Angeles and San Diego § Truvada® as part of a combination prevention package with enhanced adherence support in the forms of counseling, text messaging, and drug levels for 135 heterosexual, cis-gender women at risk of HIV infection § 5 sites (4 in LAC, 1 in SD) § Estimated enrollment beginning ~late April 2016

Pr. EP Awareness, Access and Roll-Out Considerations for Advocacy and Policy David Evans, Director of Research Advocacy

Disclosures • Project Inform receives restricted and unrestricted funding from pharmaceutical companies, including Gilead. • Today’s educational forum and presentation have been created independently of pharmaceutical influence or review.

About Project Inform • An HIV and Hepatitis C advocacy, education and policy organizations that has been in existence since 1985. • Work on biomedical prevention began in 2011, with calls for demonstration projects to determine real world efficacy and more recently work on implementation of Pr. EP programs in California and elsewhere. • Project Inform is one of the oldest organizations working on pricing of and access to HIV medications both for those living with HIV and now those at risk of HIV as well.

Over-all topics for today: • EFFECTIVENESS: What have we learned about the effectiveness of Pr. EP in men who have sex with men (MSM) and cis-gender heterosexual women and men? • DOSING: How adherent does someone need to be? How long must you take it for it to be effective and how long do you need to keep taking it after risk stops? • ACCEPTANCE AND AWARENESS: What do various communities think and know about Pr. EP and how might that affect its use? What don’t we know? • ROLL-OUT: How is Pr. EP being taken up? What are barriers to access? Are they changing? • NEW STRATEGIES: What might Pr. EP look like in 2020? New drugs, new delivery methods.

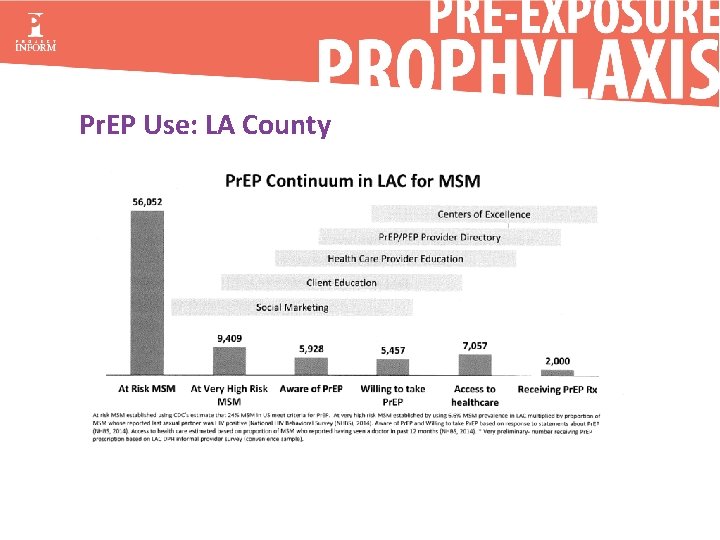
Pr. EP Use: LA County

AWARENESS and ACCEPTANCE: Presentation by Kevin Delaney of CDC at CROI 2016 on Pr. EP awareness, acceptability and use among MSM in the United States. • Two surveys: May to August 2012 (n=2, 794) and October 2014 to May 2015 (n=8, 406) • Awareness increased from 45% to 68% • Willingness to consider increased from 39% to 50% • Use increased from 0. 5% to 4. 9%

AWARENESS and ACCEPTANCE: • Hypothetical acceptability is a somewhat poor predictor of uptake of new innovations, but can prompt the need for research. • First Pr. EP study exclusively in black MSM reported at CROI 2016 (HPTN 073). • 226 men (40% under 25 yrs) offered Pr. EP. • Most (79%) decided to take it. 68% remained on Pr. EP for at least 26 weeks. • Periodic surveys among MSM in NYC have found an increase of Pr. EP use over time, starting at 2% in 2013 to 14. 8% in 2015. Race did not affect likelihood of Pr. EP use, nor did age or income, but increased risk increased it. Being uninsured reduced the likelihood of Pr. EP use.

AWARENESS and ACCEPTANCE: • Three surveys or focus groups among cis-gender women indicated moderate levels of acceptance of Pr. EP, though more studies are needed. • Data in trans women and MSM are greatly needed. Neither mentioned in CDC guidance. Concerns reported over interactions with gender conforming hormones and Pr. EP and effect on genital tissues. • Almost no work has been done around persons who inject drugs.

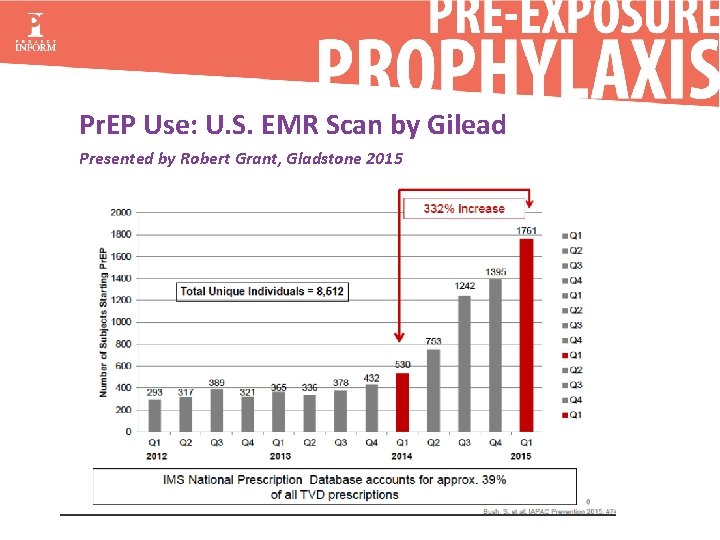
Pr. EP Use: U. S. EMR Scan by Gilead Presented by Robert Grant, Gladstone 2015

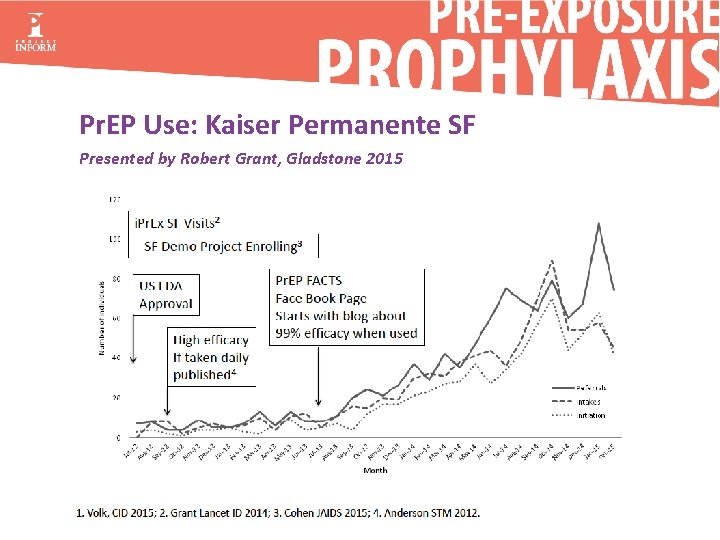
Pr. EP Use: Kaiser Permanente SF Presented by Robert Grant, Gladstone 2015

ROLL-OUT: • Some factors influencing access: • Stigma from friends, the community and providers • Lack of health insurance • Incomplete health insurance with high deductibles and co-pays • Lack of knowledgeable and willing providers • Lack of access to regular STI and lab tests • Low perception of HIV risk

ROLL-OUT: • Signs of improvement: • Increased co-pay assistance • New Pr. EP clinics and Pr. EP services expanding even within cities with low resources • More money for Pr. EP from local, state and federal government • More culturally competent educational resources • Greater recognition of the need to expand existing Pr. EP services. • Successful state budget request for Pr. EP navigation and other services

ROLL-OUT: Other challenges and questions • Continued disagreement about who should deliver Pr. EP services. • ID and HIV specialists or PCP and NPs? • Other task shifting? • Pharmacists? • Ensuring the absence of acute infections (access to 4 th generation AB/AG test) • Proper levels of adherence and prevention support (Scale up, scale down, scrape by with limited resources? ) • Co-location or collaborative agreement with support services?

Policy opportunities and discussions: • Follow NY Pr. EP-DAP model in California – free testing and medical visits – Gilead, PAN and PAF pay for drugs. • Expanded support for rural and low-resource areas that allows flexibility while maintaining high standards. • Clearinghouse for Pr. EP education, implementation and medical practice best practices. • Education that Ryan White infrastructure can be used for non-funded services (e. g. RW admin can support Pr. EP, which is not RW funded) • Changing Ryan White to allow HIV prevention

Materials from Project Inform • We have 5 printed materials on Pr. EP (order free copies): • Is Pr. EP the right choice for you? (for both cisgender and transgender MSM) • Pr. EP: A new option for women for safer loving How to get Pr. EP • Transcending barriers for safer pleasure (for transgender women) • Pr. EP Flow Chart (an access guide to obtaining a prescription and coverage for Pr. EP) • Pocket point of access card www. projectinform. org/prep

Resources Clinical Recommendations, Guidelines and Tools LA County http: //publichealth. lacounty. gov/dhsp/docs/Pr. EPService. Delivery. Checklist. Providers. pdf http: //publichealth. lacounty. gov/dhsp/docs/LACounty. PEP-Provider. Directory 515. pdf CDC http: //www. cdc. gov/hiv/guidelines/preventing. html Medication and Co-Pay Assistance https: //www. gileadadvancingaccess. com/copay-coupon-card https: //www. gileadadvancingaccess. com/get-started-advancing-access https: //www. copays. org/diseases/hiv-aids-and-prevention Provider Directory for LA County www. Get. Pr. EPLA. com http: //getprepla. com/provider_directory. html. Patient Support and Resourceswww. Prep. Facts. org

Contact Us Janie Caplan, MD – David Geffen School of Medicine at UCLA Email: mcaplan@mednet. ucla. edu Phone: 866 -562 -1048 (AEGi. S) Gifty-Maria Ntim, MD, MPH – APLA Health & Wellness Email: gntim@apla. org Phone: 323. 329. 9929 David Evans, Project Inform Email: devans@projectinform. org Phone: 626 -241 -8267