Preparation of PolymerSilica Particle Nanocomposites and Their Applications
















![Green Tires How can the Silane coupling agent meet the needs? Si-69; Bis-[-3 -(triethoxysilyl)propyl]-tetrasulfide Green Tires How can the Silane coupling agent meet the needs? Si-69; Bis-[-3 -(triethoxysilyl)propyl]-tetrasulfide](https://slidetodoc.com/presentation_image_h/c236aa4c1911d8de5556ca889449e127/image-41.jpg)




![Experimental Materials: 1) Silica OX-50 & Aerosil 380 2) Ɣ-methacryloxypropyltrichlorosilane (MPTS) 3) [(Biscycloheptenyl)ethyl]tricholorosilane (BCTC) Experimental Materials: 1) Silica OX-50 & Aerosil 380 2) Ɣ-methacryloxypropyltrichlorosilane (MPTS) 3) [(Biscycloheptenyl)ethyl]tricholorosilane (BCTC)](https://slidetodoc.com/presentation_image_h/c236aa4c1911d8de5556ca889449e127/image-51.jpg)












- Slides: 82

Preparation of Polymer/Silica Particle Nanocomposites and Their Applications (고분자/실리카 나노복합체 제조와 응용) Nov. 5, 2009 Ki. Ryong Ha (계명대학교 화학공학과) 2020 -11 -30 1/82

Colorado 주 및 Colorado 대학 소개 2020 -11 -30 2/82

University of Colorado Three campuses: Boulder, Colorado Springs and Denver. 2020 -11 -30 3/82

National Parks in Colorado Mesa Verde National Park 2020 -11 -30 Rocky Mountain National Park 4/82

National Parks in Colorado Great Sand Dunes National Park 2020 -11 -30 Black Canyon of the Gunnison National Park 5/82

Tourist Attractions Aspen Colorado Ski Resort 2020 -11 -30 Aspen mountains comprises of 4993 acres, forty lifts and 335 trails along with sharp vertical slopes in the entire Colorado, which makes it more thrilling and stimulating. 6/82

Tourist Attractions Maroon Bells 2020 -11 -30 One Colorado Fall day 7/82

Tourist Attractions Garden of the gods 2020 -11 -30 United States Air Force Academy 8/82

University of Colorado at Boulder 2020 -11 -30 9/82

Department of Chemical and Biological Engineering Our department has been ranked 19 th overall and 10 th among public graduate programs by U. S. News & World Report, and ranked 4 th in average citations per publication by University Science Indicators. Dr. Christopher N. Bowman Associate Dean for Research, Patten Professor of Chemical and Biological Engineering, Clinical Professor of Restorative Dentistry and Co-Director of the NSF I/UCRC for Fundamentals and Applications and Photopolymerizations 2020 -11 -30 10/82

Outlines 1. Introduction - Composites and Nanocomposites - Silica & Silane Coupling Agent 2. Experimental - Silanization of Silica Particles - Characterization (a) FTIR (b) TGA (c) Solid State NMR - Fabrication of Nanocomposites (a) Curing Kinetics using Real Time NIR (b) DMA results 3. Conclusions 2020 -11 -30 11/82

Introduction 2020 -11 -30 12/82

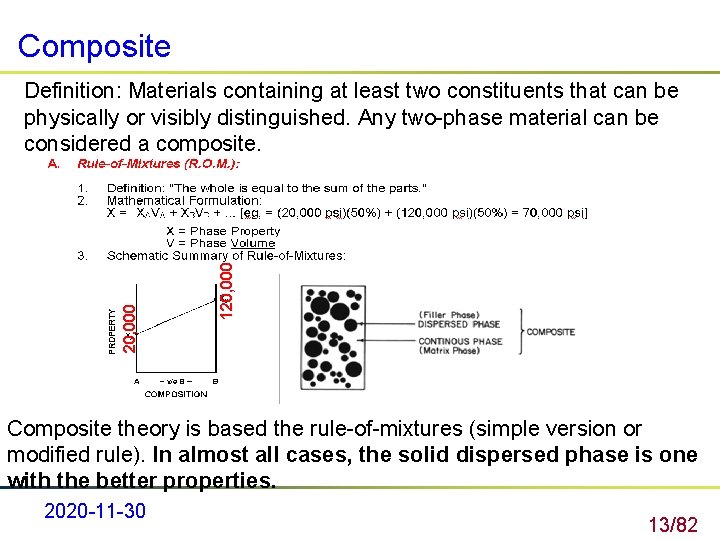
Composite 120, 000 Definition: Materials containing at least two constituents that can be physically or visibly distinguished. Any two-phase material can be considered a composite. Composite theory is based the rule-of-mixtures (simple version or modified rule). In almost all cases, the solid dispersed phase is one with the better properties. 2020 -11 -30 13/82

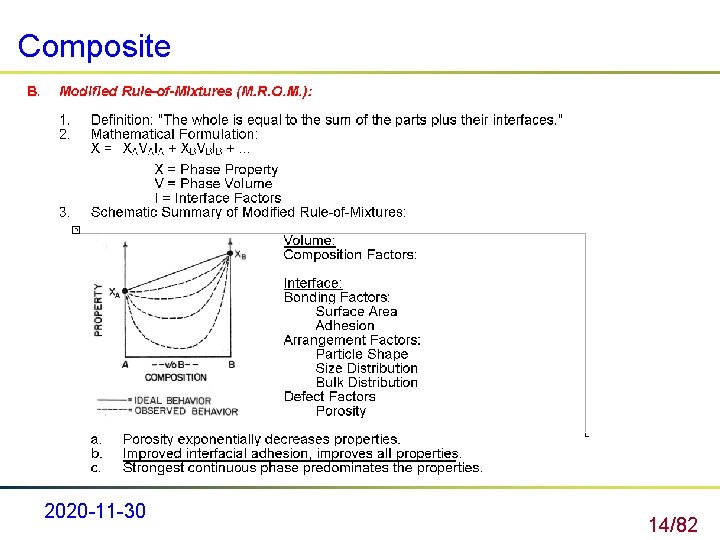
Composite 2020 -11 -30 14/82

Effect of Fillers Functional fillers transfer applied stress from the polymer matrix to the strong and stiff mineral. 2020 -11 -30 15/82

Polymer Nanocomposites 2020 -11 -30 16/82

Polymer Matrices 2020 -11 -30 17/82

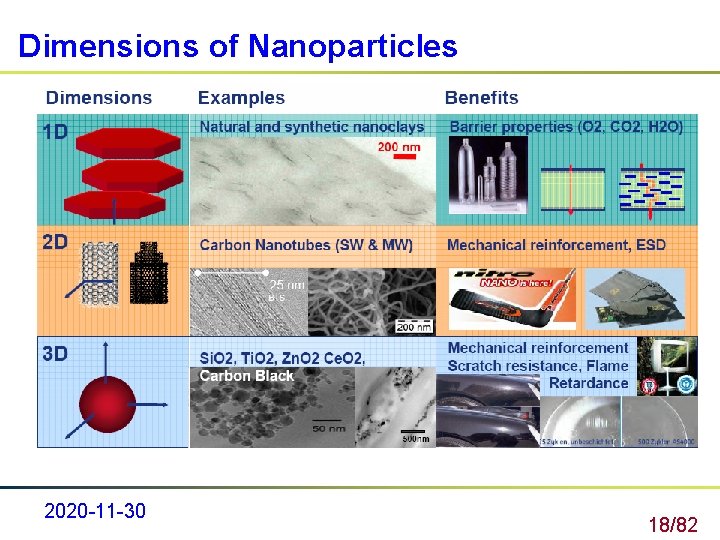
Dimensions of Nanoparticles 2020 -11 -30 18/82

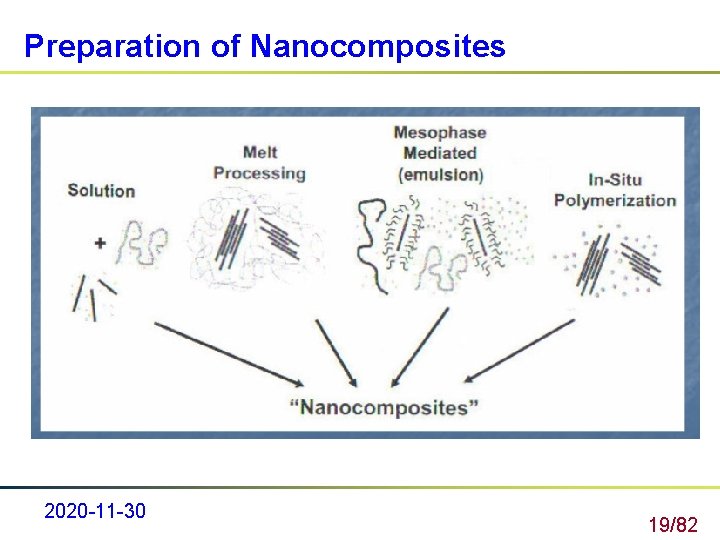
Preparation of Nanocomposites 2020 -11 -30 19/82

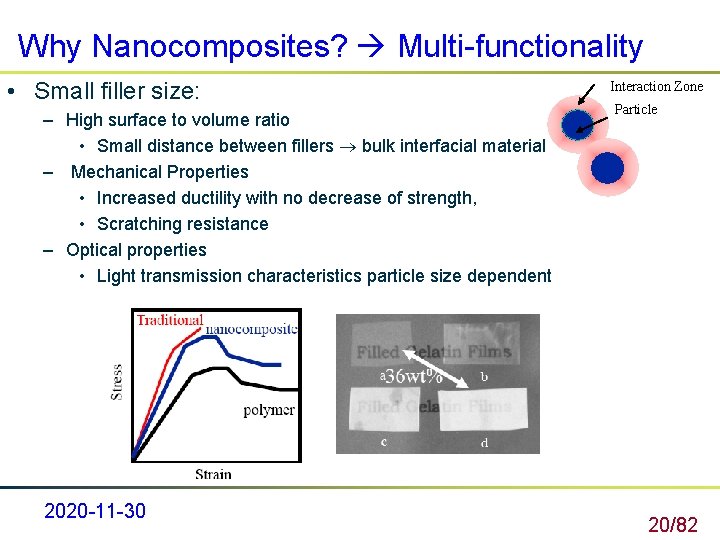
Why Nanocomposites? Multi-functionality • Small filler size: – High surface to volume ratio • Small distance between fillers bulk interfacial material – Mechanical Properties • Increased ductility with no decrease of strength, • Scratching resistance – Optical properties • Light transmission characteristics particle size dependent 2020 -11 -30 Interaction Zone Particle 20/82

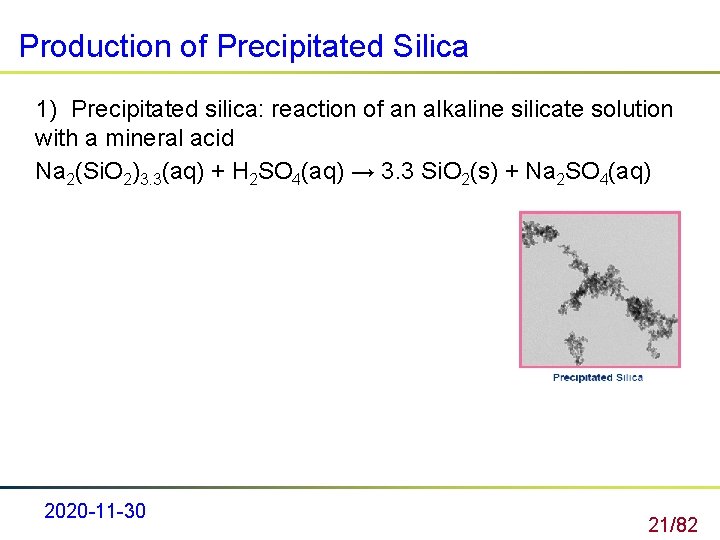
Production of Precipitated Silica 1) Precipitated silica: reaction of an alkaline silicate solution with a mineral acid Na 2(Si. O 2)3. 3(aq) + H 2 SO 4(aq) → 3. 3 Si. O 2(s) + Na 2 SO 4(aq) 2020 -11 -30 21/82

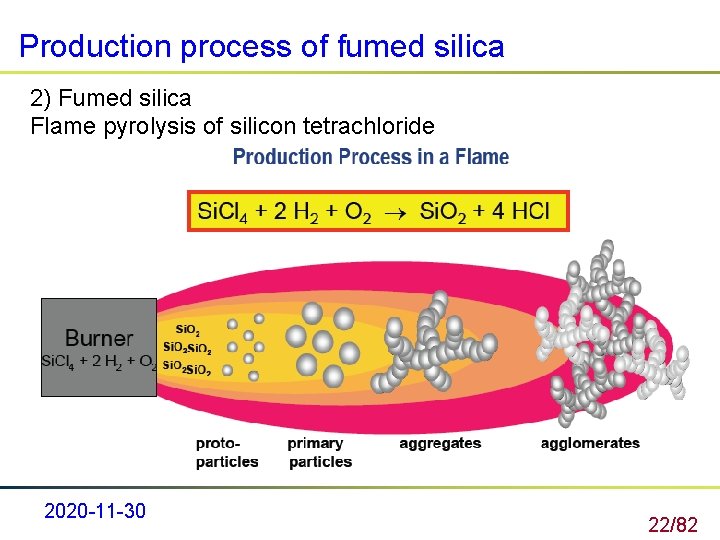
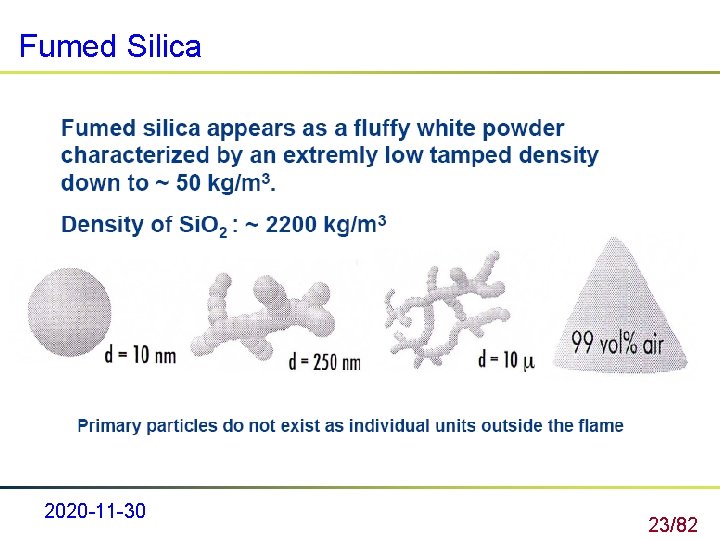
Production process of fumed silica 2) Fumed silica Flame pyrolysis of silicon tetrachloride 2020 -11 -30 22/82

Fumed Silica 2020 -11 -30 23/82

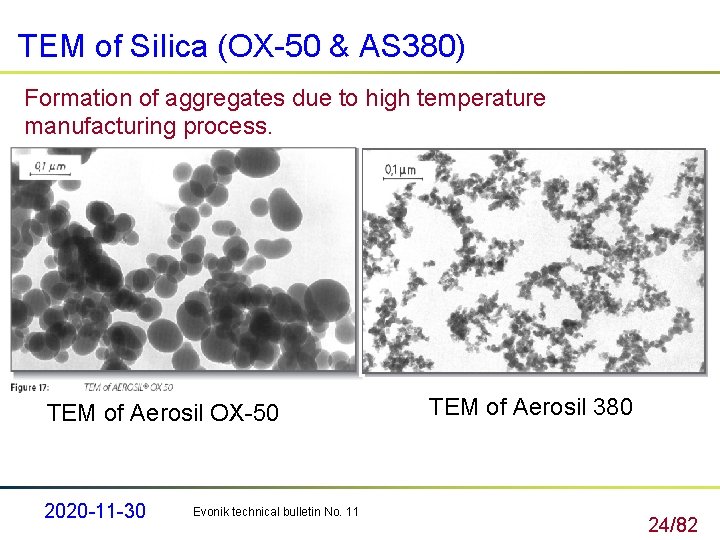
TEM of Si. Iica (OX-50 & AS 380) Formation of aggregates due to high temperature manufacturing process. TEM of Aerosil OX-50 2020 -11 -30 Evonik technical bulletin No. 11 TEM of Aerosil 380 24/82

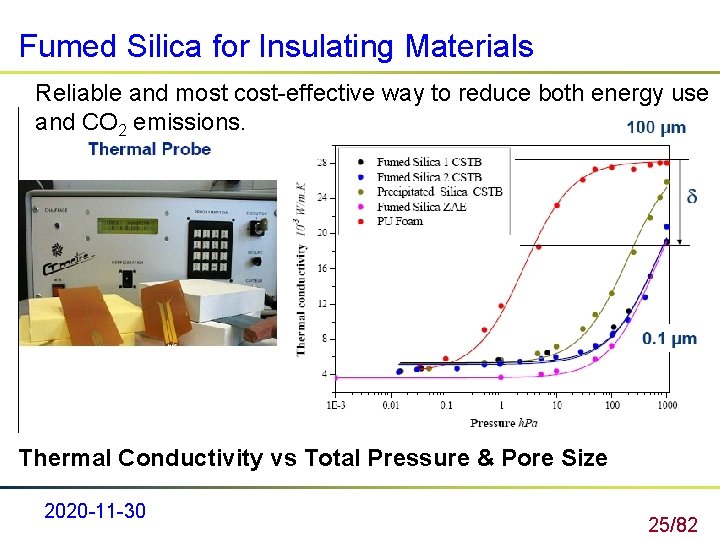
Fumed Silica for Insulating Materials Reliable and most cost-effective way to reduce both energy use and CO 2 emissions. Thermal Conductivity vs Total Pressure & Pore Size 2020 -11 -30 25/82

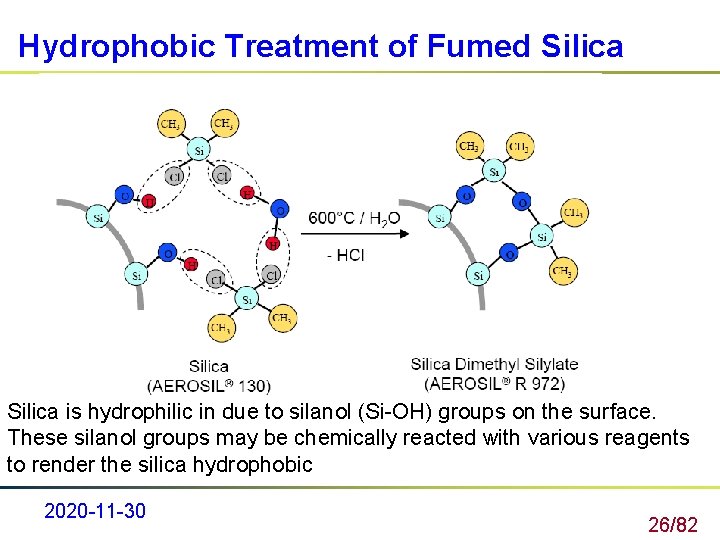
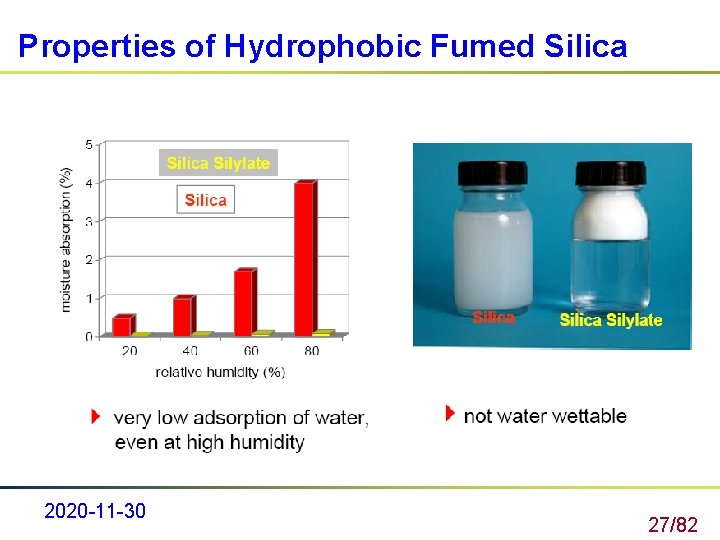
Hydrophobic Treatment of Fumed Silica is hydrophilic in due to silanol (Si-OH) groups on the surface. These silanol groups may be chemically reacted with various reagents to render the silica hydrophobic 2020 -11 -30 26/82

Properties of Hydrophobic Fumed Silica 2020 -11 -30 27/82

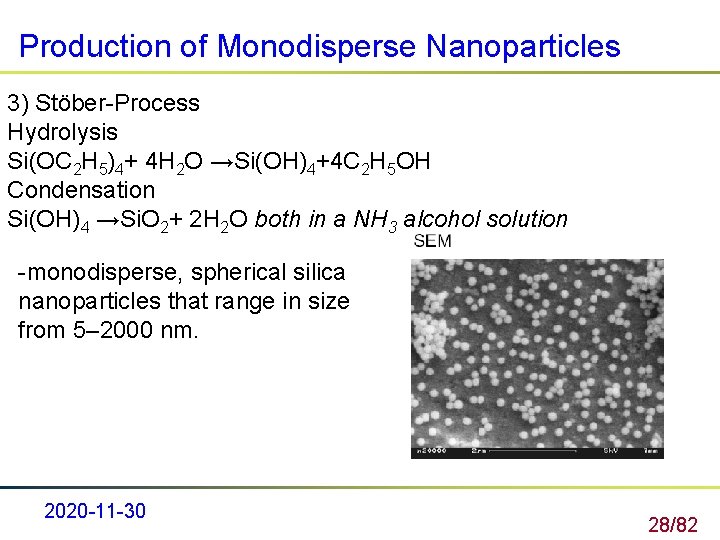
Production of Monodisperse Nanoparticles 3) Stöber-Process Hydrolysis Si(OC 2 H 5)4+ 4 H 2 O →Si(OH)4+4 C 2 H 5 OH Condensation Si(OH)4 →Si. O 2+ 2 H 2 O both in a NH 3 alcohol solution -monodisperse, spherical silica nanoparticles that range in size from 5– 2000 nm. 2020 -11 -30 28/82

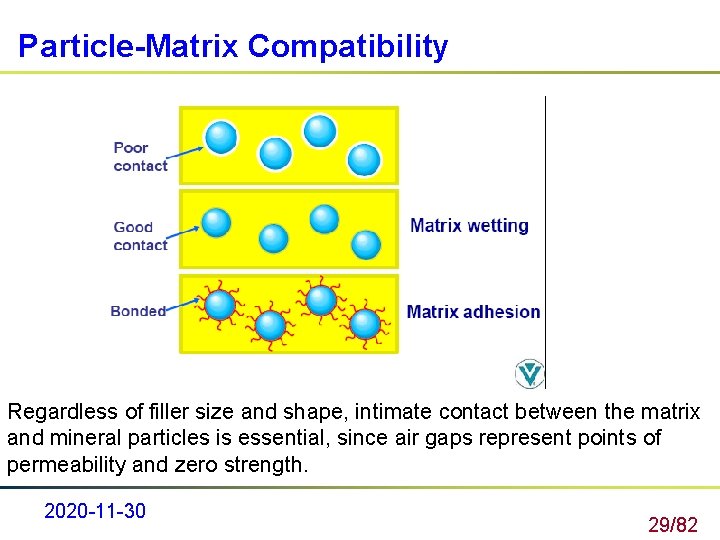
Particle-Matrix Compatibility Regardless of filler size and shape, intimate contact between the matrix and mineral particles is essential, since air gaps represent points of permeability and zero strength. 2020 -11 -30 29/82

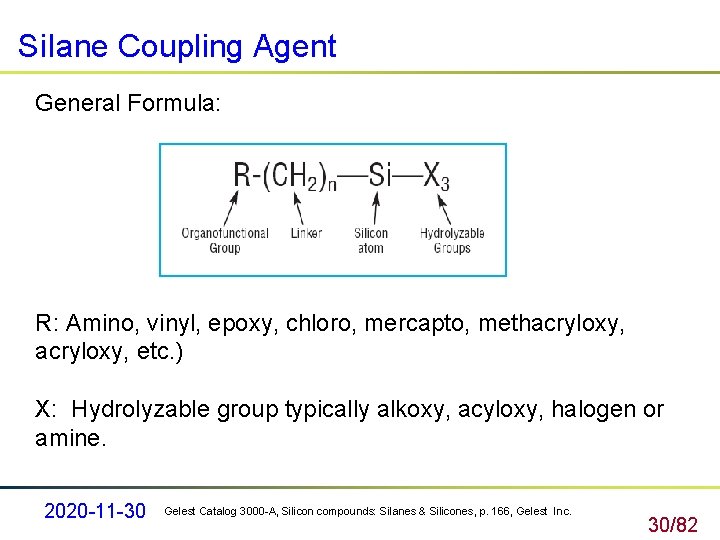
Si. Iane Coupling Agent General Formula: R: Amino, vinyl, epoxy, chloro, mercapto, methacryloxy, etc. ) X: Hydrolyzable group typically alkoxy, acyloxy, halogen or amine. 2020 -11 -30 Gelest Catalog 3000 -A, Silicon compounds: Silanes & Silicones, p. 166, Gelest Inc. 30/82

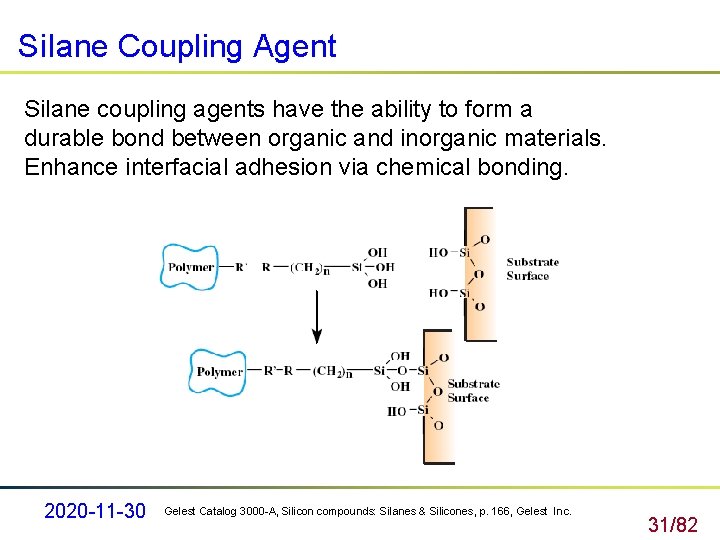
Si. Iane Coupling Agent Silane coupling agents have the ability to form a durable bond between organic and inorganic materials. Enhance interfacial adhesion via chemical bonding. 2020 -11 -30 Gelest Catalog 3000 -A, Silicon compounds: Silanes & Silicones, p. 166, Gelest Inc. 31/82

Silane Coupling agent Treatment Modification with organosilane depends on the ability to form a bond with silanol groups, -Si-OH, and/or aluminol groups (-Al-OH) on the filler surface. 2020 -11 -30 32/82

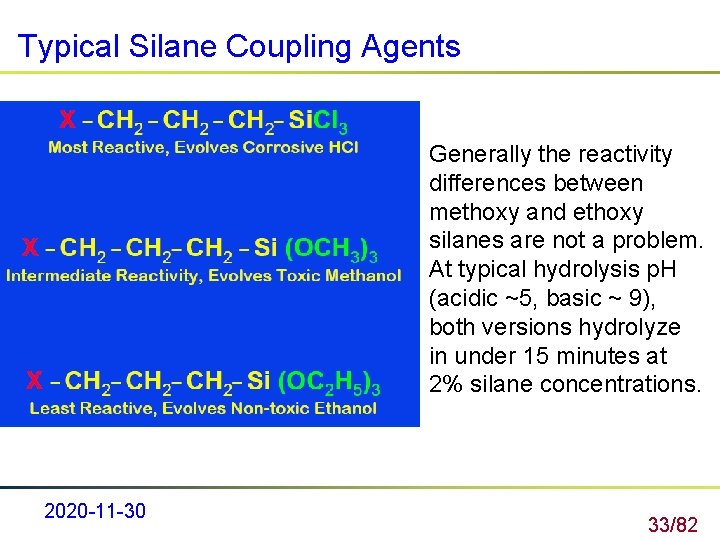
Typical Silane Coupling Agents Generally the reactivity differences between methoxy and ethoxy silanes are not a problem. At typical hydrolysis p. H (acidic ~5, basic ~ 9), both versions hydrolyze in under 15 minutes at 2% silane concentrations. 2020 -11 -30 33/82

Applying a Silane Coupling Agent 2020 -11 -30 34/82

Silane Effectiveness on Inorganics Hydroxyl-containing substrates vary widely in concentration and type of hydroxyl groups present. 2020 -11 -30 35/82

Silane Coupling agent Treatment SEM photomicrographs of fractured silica-filled epoxy composite a) silica without silane treatment, b) silica with silane treatment. 2020 -11 -30 36/82

CO 2 Reduction 2020 -11 -30 37/82

Established Nanotechnologies 2020 -11 -30 38/82

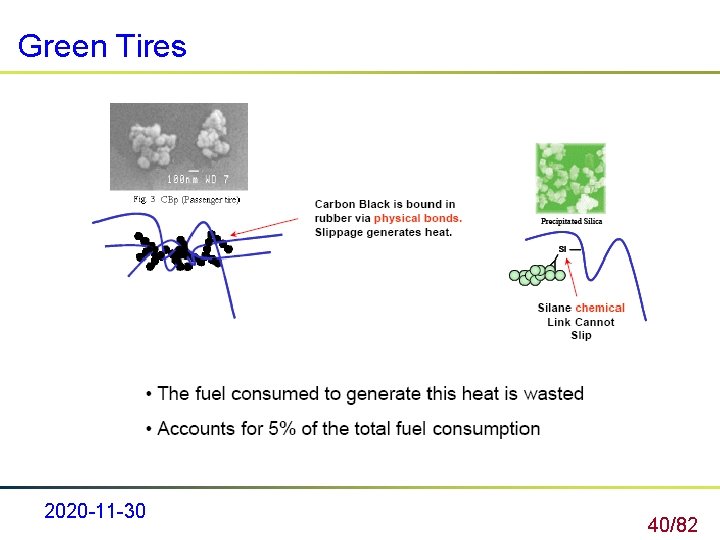
Green Tires – Lower rolling resistance – Fuel economy lower carbon dioxide emissions lower global warming impact 2020 -11 -30 39/82

Green Tires 2020 -11 -30 40/82
![Green Tires How can the Silane coupling agent meet the needs Si69 Bis3 triethoxysilylpropyltetrasulfide Green Tires How can the Silane coupling agent meet the needs? Si-69; Bis-[-3 -(triethoxysilyl)propyl]-tetrasulfide](https://slidetodoc.com/presentation_image_h/c236aa4c1911d8de5556ca889449e127/image-41.jpg)
Green Tires How can the Silane coupling agent meet the needs? Si-69; Bis-[-3 -(triethoxysilyl)propyl]-tetrasulfide 2020 -11 -30 41/82

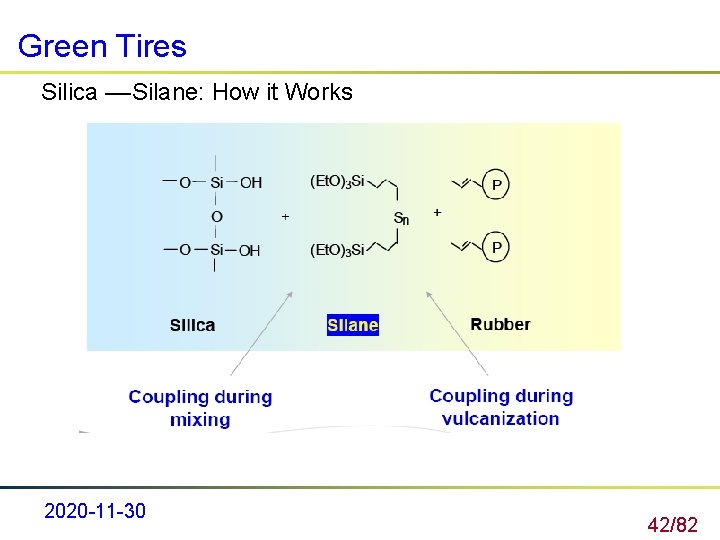
Green Tires Silica ––Silane: How it Works 2020 -11 -30 42/82

Green Tires Silica ––Silane: How it Works 2020 -11 -30 43/82

Green Tires – How it works 2020 -11 -30 44/82

Silanes to Meet the Need 2020 -11 -30 45/82

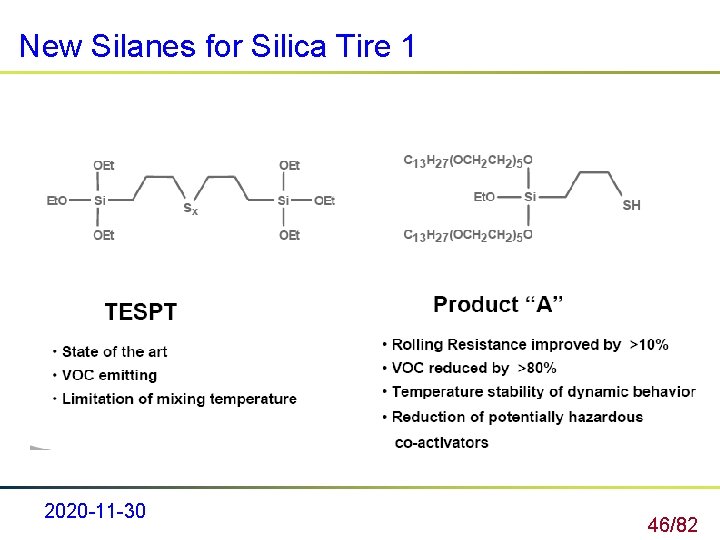
New Silanes for Silica Tire 1 2020 -11 -30 46/82

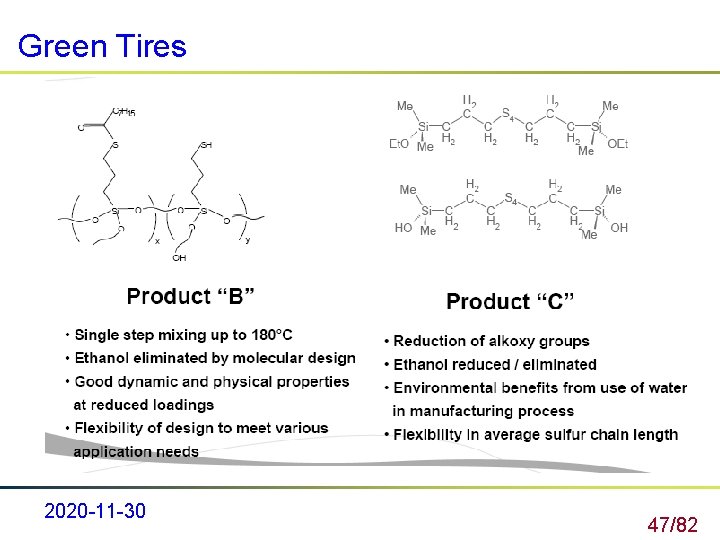
Green Tires 2020 -11 -30 47/82

Experimental 2020 -11 -30 48/82

Properties of Si. Iica (OX-50 & Aerosil 380) 1) Noncrystalline form of silicon dioxide (Si. O 2) – Fumed silica - OX-50: Low specific surface and only slight tendency to agglomerate. ( 2. 2 Si-OH/nm 2) - Aerosil 380: Highest specific surface area (2. 5 Si-OH/nm 2) - Hydrophilic grades. 2) BET Surface Area [m 2/g]: 50 (+-) 15, 380 (+-) 30 3) Average primary particle size: 40 nm, 7 nm 4) Tapped density: 130 g/L, 50 g/L 5 Density: 2. 2 g/cm 3 6) Hardness: 5. 3– 6. 5 (Mohs Scale) Fillers for transparent scratch-resistant coating 7) Refractive index: 1. 46 – Very close to the most organic monomers 8) Tensile strength: 48. 3 MPa 9) Bulk modulus: ~37 Gpa 10) Young’s modulus: 71. 7 GPa Fillers for reinforcing 2020 -11 -30 49/82

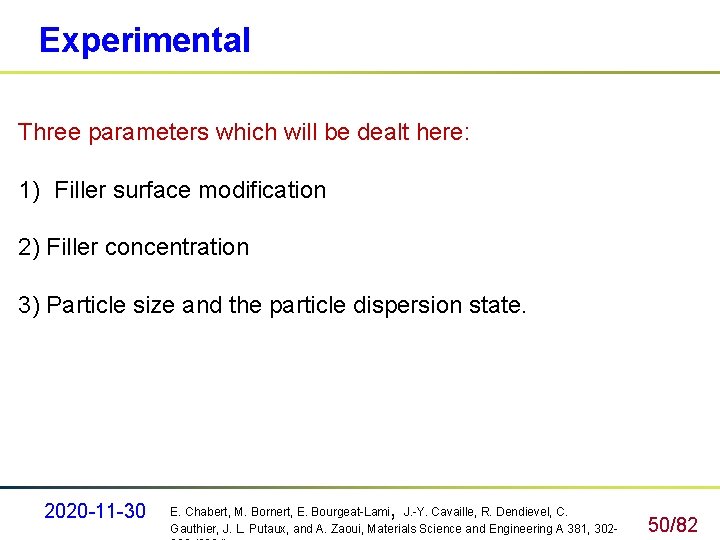
Experimental Three parameters which will be dealt here: 1) Filler surface modification 2) Filler concentration 3) Particle size and the particle dispersion state. 2020 -11 -30 , E. Chabert, M. Bornert, E. Bourgeat-Lami J. -Y. Cavaille, R. Dendievel, C. Gauthier, J. L. Putaux, and A. Zaoui, Materials Science and Engineering A 381, 302 - 50/82
![Experimental Materials 1 Silica OX50 Aerosil 380 2 Ɣmethacryloxypropyltrichlorosilane MPTS 3 Biscycloheptenylethyltricholorosilane BCTC Experimental Materials: 1) Silica OX-50 & Aerosil 380 2) Ɣ-methacryloxypropyltrichlorosilane (MPTS) 3) [(Biscycloheptenyl)ethyl]tricholorosilane (BCTC)](https://slidetodoc.com/presentation_image_h/c236aa4c1911d8de5556ca889449e127/image-51.jpg)
Experimental Materials: 1) Silica OX-50 & Aerosil 380 2) Ɣ-methacryloxypropyltrichlorosilane (MPTS) 3) [(Biscycloheptenyl)ethyl]tricholorosilane (BCTC) 4) TMPTMP (Trimethylolpropane tris(3 -mercaptopropionate) -3 functional S-H -n 20 = 1. 518, d=1. 21 g/ml -b. p. = 220 ℃ at 0. 3 mm Hg, m. w. = 398. 56 5) TMPDE (Trimethylolpropane diallyl ether) -2 functional allyl ether groups -n. D =1. 458, d= 0. 955 g/ml -b. p. = 135℃/13 mm. Hg, m. w. = 214. 30 2020 -11 -30 51/82

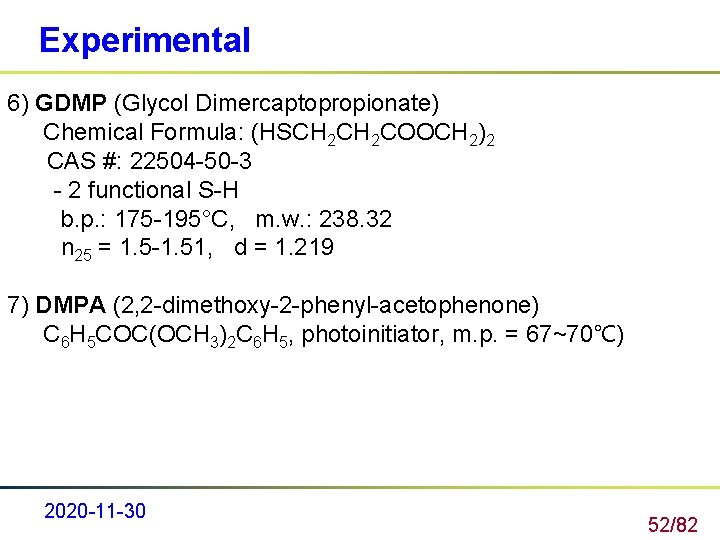
Experimental 6) GDMP (Glycol Dimercaptopropionate) Chemical Formula: (HSCH 2 COOCH 2)2 CAS #: 22504 -50 -3 - 2 functional S-H b. p. : 175 -195°C, m. w. : 238. 32 n 25 = 1. 5 -1. 51, d = 1. 219 7) DMPA (2, 2 -dimethoxy-2 -phenyl-acetophenone) C 6 H 5 COC(OCH 3)2 C 6 H 5, photoinitiator, m. p. = 67~70℃) 2020 -11 -30 52/82

Silane treatment procedure (Dean-Stark trap) To make monolayer of silane coupling agent on the silica, water must be removed from the reaction system. 1) Silica particles were dried by heating under vacuum 2) Dean-Stark trap was used to remove water in the toluene solvent. 3) Dried silica particles were added to the reaction flask with dried toluene. 4) Silane solution and triethylamine (catalyst) were added and stirred under nitrogen for 18 hrs. 5) The particles were washed with several kinds of solvents and dried in the vacuum oven. 2020 -11 -30 53/82

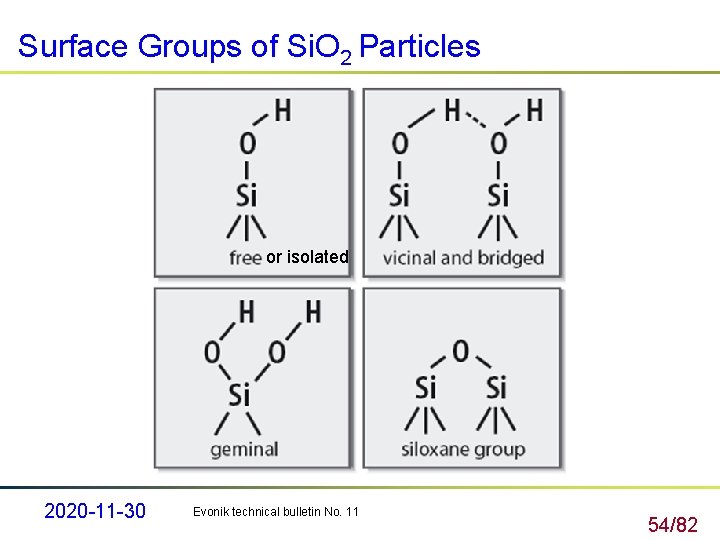
Surface Groups of Si. O 2 Particles or isolated 2020 -11 -30 Evonik technical bulletin No. 11 54/82

Drying of OX-50 (FTIR Spectrum of of Pressed Disks) Pressed OX-50 without any treatment Before heating After 1 hr heating at 150℃ By drying process, increase of the intensity at 3747 cm-1, which is caused by isolated Si-OH was observed (150℃ for 1 hr). 2020 -11 -30 55/82

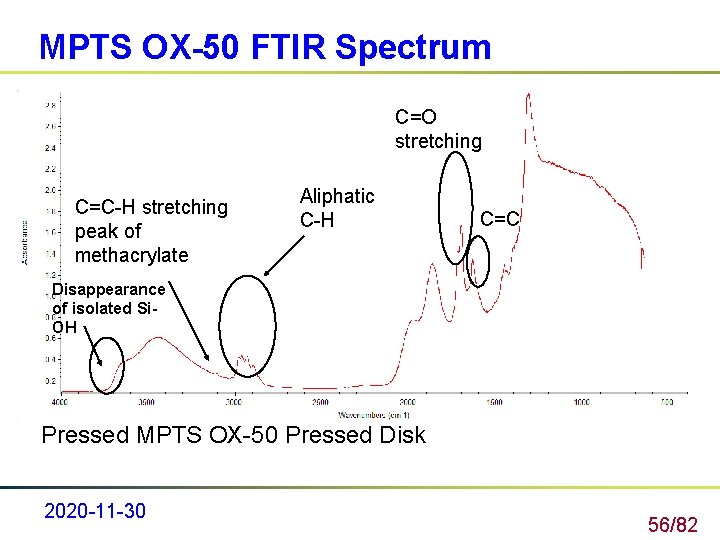
MPTS OX-50 FTIR Spectrum C=O stretching C=C-H stretching peak of methacrylate Aliphatic C-H C=C Disappearance of isolated Si. OH Pressed MPTS OX-50 Pressed Disk 2020 -11 -30 56/82

FTIR Results (C=O Peak Change) H-bonded C=O Free C=O C=C (c) (b) (a): Pure MPTS liquid, (b): Anhydrous toluene was used to prepare (multilayer), (c) Reflux method was used to prepare. 2020 -11 -30 Q. Liu et al. , JOURNAL OF BIOMEDICAL MATERIALS RESEARCH 57(3), 384 -393 (2001). 57/82

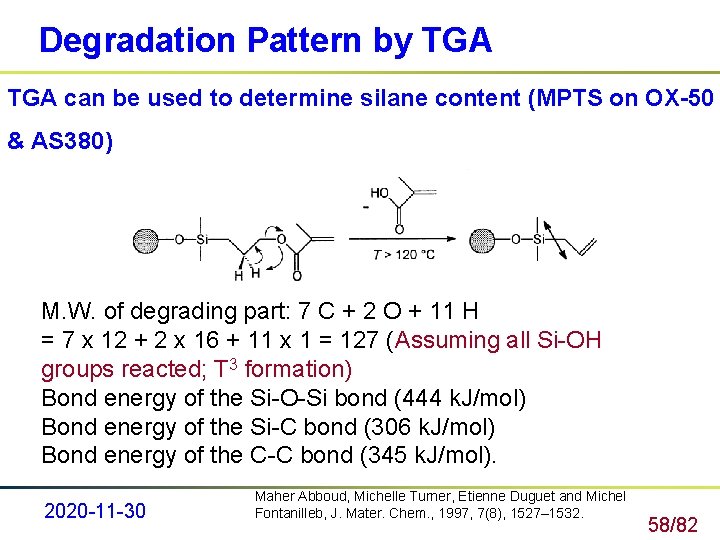
Degradation Pattern by TGA can be used to determine silane content (MPTS on OX-50 & AS 380) M. W. of degrading part: 7 C + 2 O + 11 H = 7 x 12 + 2 x 16 + 11 x 1 = 127 (Assuming all Si-OH groups reacted; T 3 formation) Bond energy of the Si-O-Si bond (444 k. J/mol) Bond energy of the Si-C bond (306 k. J/mol) Bond energy of the C-C bond (345 k. J/mol). 2020 -11 -30 Maher Abboud, Michelle Turner, Etienne Duguet and Michel Fontanilleb, J. Mater. Chem. , 1997, 7(8), 1527– 1532. 58/82

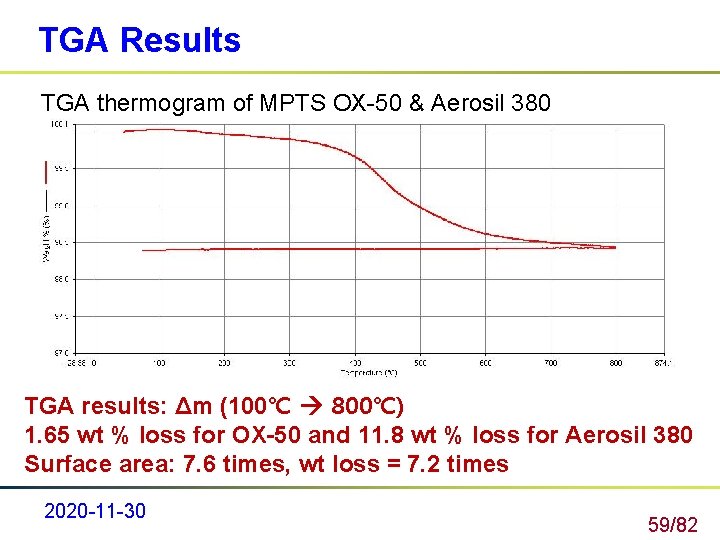
TGA Results TGA thermogram of MPTS OX-50 & Aerosil 380 TGA results: Δm (100℃ 800℃) 1. 65 wt % loss for OX-50 and 11. 8 wt % loss for Aerosil 380 Surface area: 7. 6 times, wt loss = 7. 2 times 2020 -11 -30 59/82

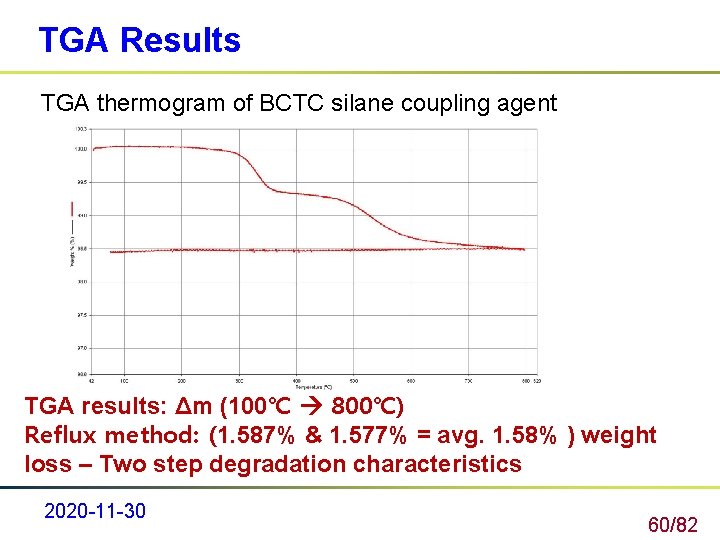
TGA Results TGA thermogram of BCTC silane coupling agent TGA results: Δm (100℃ 800℃) Reflux method: (1. 587% & 1. 577% = avg. 1. 58% ) weight loss – Two step degradation characteristics 2020 -11 -30 60/82

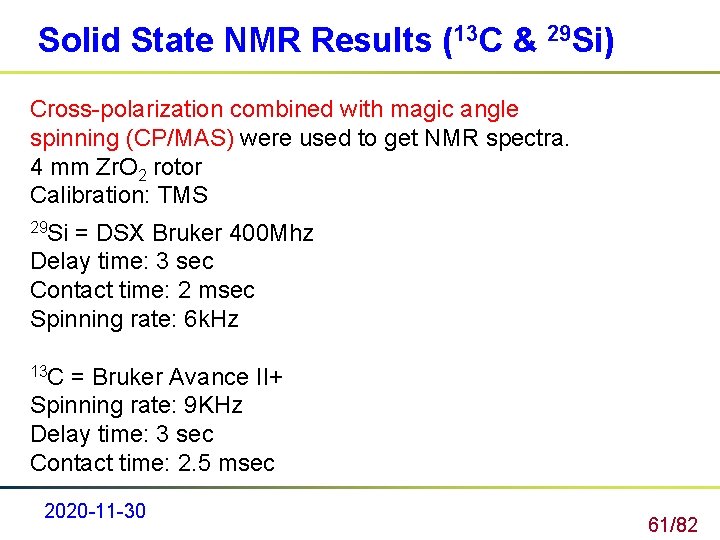
Solid State NMR Results (13 C & 29 Si) Cross-polarization combined with magic angle spinning (CP/MAS) were used to get NMR spectra. 4 mm Zr. O 2 rotor Calibration: TMS 29 Si = DSX Bruker 400 Mhz Delay time: 3 sec Contact time: 2 msec Spinning rate: 6 k. Hz 13 C = Bruker Avance II+ Spinning rate: 9 KHz Delay time: 3 sec Contact time: 2. 5 msec 2020 -11 -30 61/82

Solid State 29 Si NMR From 29 Si CP/MAS NMR, it is possible to differentiate the different types of silicon atoms present in the silica particles: Q 4, Q 3, and Q 2, that is, in the bulk, on the surface bonded to one OH and to two OH, respectively. T 1, T 2, and T 3 correspond to the silicon atoms contained in the silane molecule which have formed one (or two, or three, respectively) Si-O-Si bond with the silica particle, or one Si-O-Si binding between two silanes. 2 2020 -11 -30 62/82

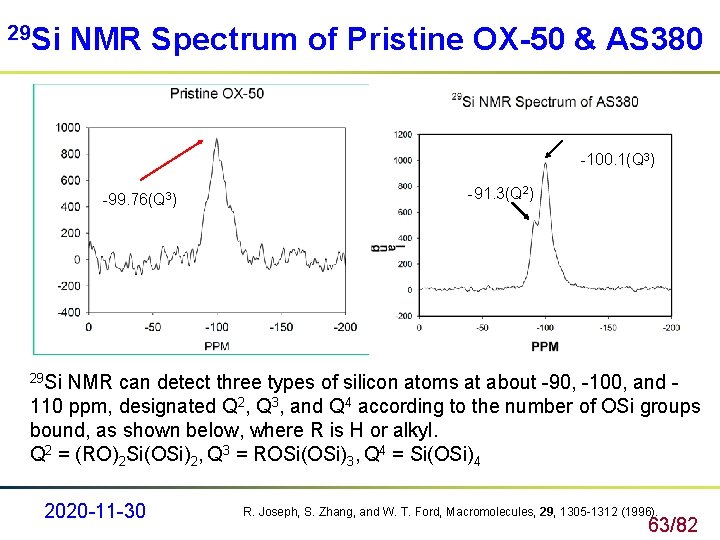
29 Si NMR Spectrum of Pristine OX-50 & AS 380 -100. 1(Q 3) -99. 76(Q 3) -91. 3(Q 2) 29 Si NMR can detect three types of silicon atoms at about -90, -100, and - 110 ppm, designated Q 2, Q 3, and Q 4 according to the number of OSi groups bound, as shown below, where R is H or alkyl. Q 2 = (RO)2 Si(OSi)2, Q 3 = ROSi(OSi)3, Q 4 = Si(OSi)4 2020 -11 -30 R. Joseph, S. Zhang, and W. T. Ford, Macromolecules, 29, 1305 -1312 (1996). 63/82

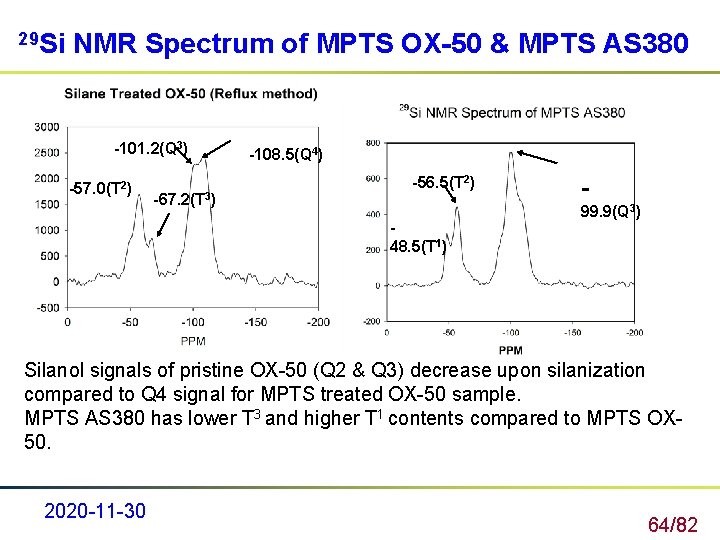
29 Si NMR Spectrum of MPTS OX-50 & MPTS AS 380 -101. 2(Q 3) -57. 0(T 2) -67. 2(T 3) -108. 5(Q 4) -56. 5(T 2) 48. 5(T 1) 99. 9(Q 3) Silanol signals of pristine OX-50 (Q 2 & Q 3) decrease upon silanization compared to Q 4 signal for MPTS treated OX-50 sample. MPTS AS 380 has lower T 3 and higher T 1 contents compared to MPTS OX 50. 2020 -11 -30 64/82

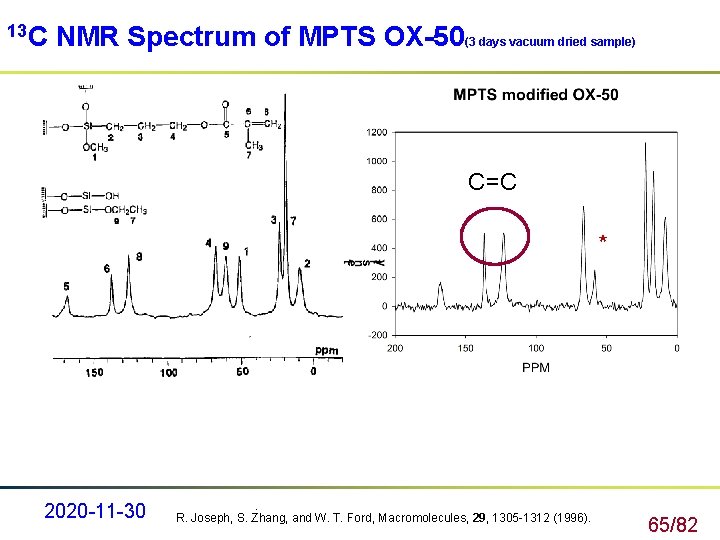
13 C NMR Spectrum of MPTS OX-50(3 days vacuum dried sample) C=C * 2020 -11 -30 . R. Joseph, S. Zhang, and W. T. Ford, Macromolecules, 29, 1305 -1312 (1996). 65/82

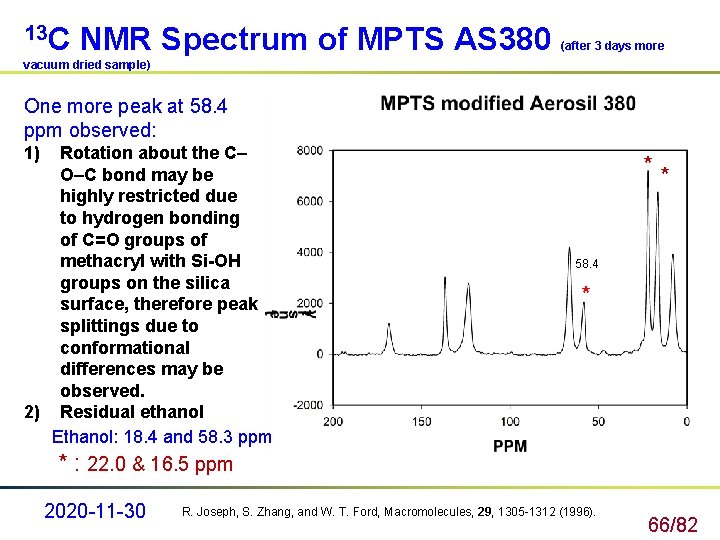
13 C NMR Spectrum of MPTS AS 380 (after 3 days more vacuum dried sample) One more peak at 58. 4 ppm observed: 1) Rotation about the C– O–C bond may be highly restricted due to hydrogen bonding of C=O groups of methacryl with Si-OH groups on the silica surface, therefore peak splittings due to conformational differences may be observed. 2) Residual ethanol Ethanol: 18. 4 and 58. 3 ppm ** 58. 4 * * : 22. 0 & 16. 5 ppm 2020 -11 -30 R. Joseph, S. Zhang, and W. T. Ford, Macromolecules, 29, 1305 -1312 (1996). 66/82

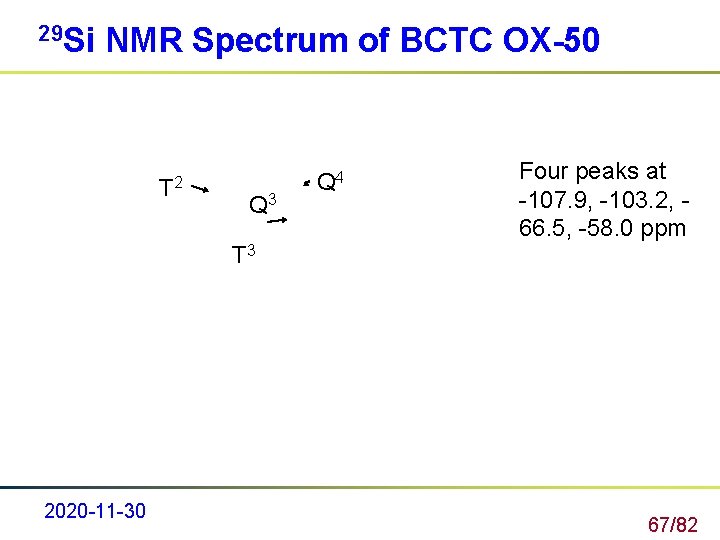
29 Si NMR Spectrum of BCTC OX-50 T 2 Q 3 T 3 2020 -11 -30 Q 4 Four peaks at -107. 9, -103. 2, 66. 5, -58. 0 ppm 67/82

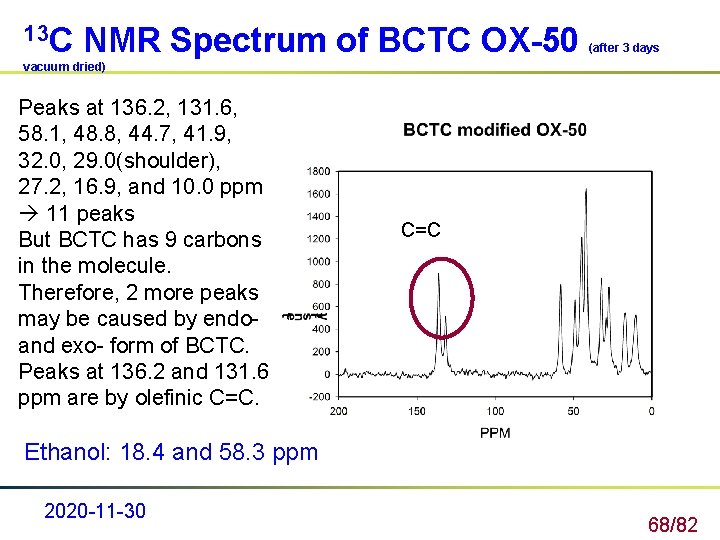
13 C NMR Spectrum of BCTC OX-50 (after 3 days vacuum dried) Peaks at 136. 2, 131. 6, 58. 1, 48. 8, 44. 7, 41. 9, 32. 0, 29. 0(shoulder), 27. 2, 16. 9, and 10. 0 ppm 11 peaks But BCTC has 9 carbons in the molecule. Therefore, 2 more peaks may be caused by endo- and exo- form of BCTC. Peaks at 136. 2 and 131. 6 ppm are by olefinic C=C Ethanol: 18. 4 and 58. 3 ppm 2020 -11 -30 68/82

Dispersion test in H 2 O 2 ml water only in the vial. OX-50 silica dispersed well in the water. Some MPTS OX-50 dispersed, but most of them in the bottom of the vial. Most BCTC OX-50 stayed in the bottom of the vial. a) MPTS OX-50 in water b) BCTC OX-50 in water c) OX-50 in water 2020 -11 -30 Hydrophilicilty OX-50 > MPTS OX-0 > BCTC OX-50 69/82

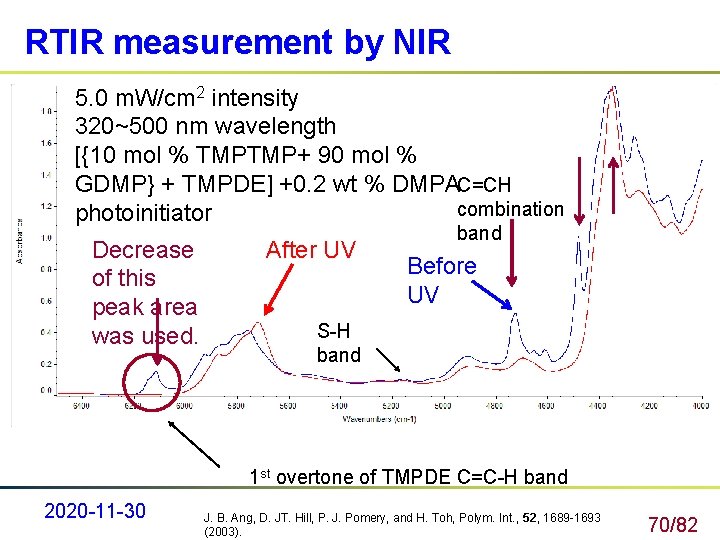
RTIR measurement by NIR 5. 0 m. W/cm 2 intensity 320~500 nm wavelength [{10 mol % TMPTMP+ 90 mol % GDMP} + TMPDE] +0. 2 wt % DMPA C=CH combination photoinitiator Decrease of this peak area was used. After UV band Before UV S-H band 1 st overtone of TMPDE C=C-H band 2020 -11 -30 J. B. Ang, D. JT. Hill, P. J. Pomery, and H. Toh, Polym. Int. , 52, 1689 -1693 (2003). 70/82

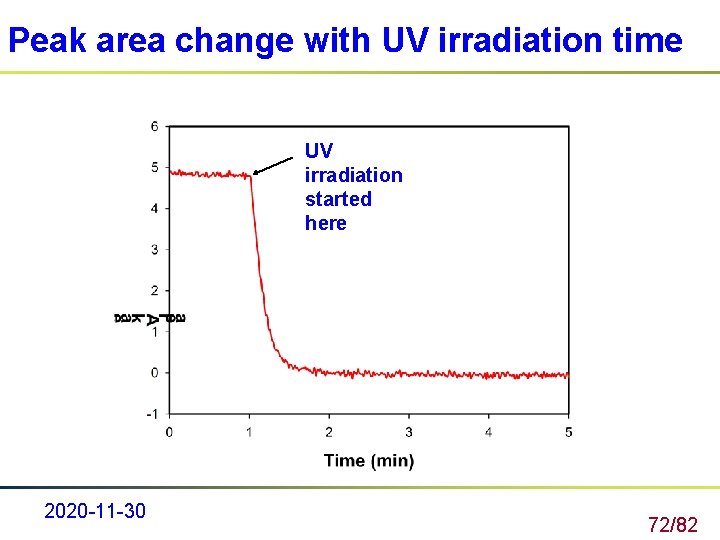
Real Time FTIR Monitoring reactions in real-time. Peak area change with UV irradiation was generated in real time, that is, the data from both UV curing and FTIR monitoring are collected simultaneously to follow the time dependent decrease of the C=C bond. 2020 -11 -30 71/82

Peak area change with UV irradiation time UV irradiation started here 2020 -11 -30 72/82

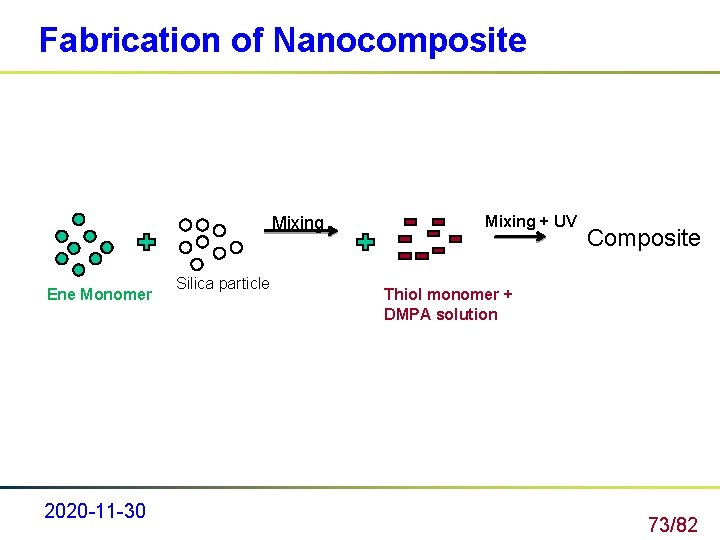
Fabrication of Nanocomposite Mixing Ene Monomer 2020 -11 -30 Silica particle Mixing + UV Composite Thiol monomer + DMPA solution 73/82

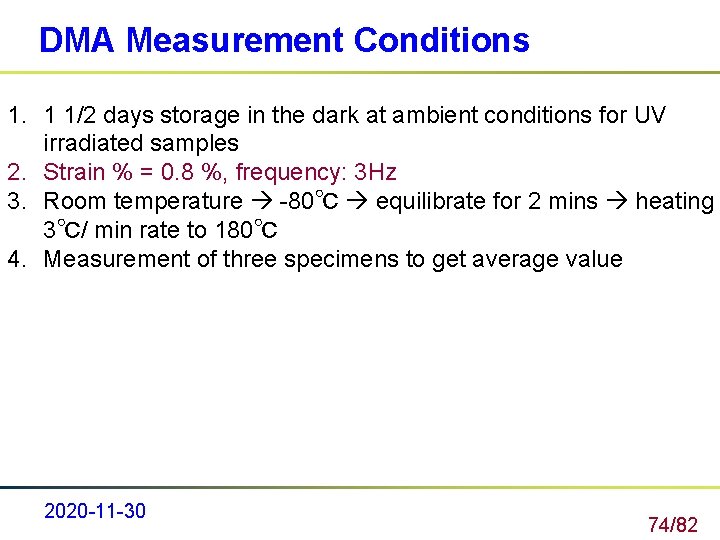
DMA Measurement Conditions 1. 1 1/2 days storage in the dark at ambient conditions for UV irradiated samples 2. Strain % = 0. 8 %, frequency: 3 Hz 3. Room temperature -80℃ equilibrate for 2 mins heating 3℃/ min rate to 180℃ 4. Measurement of three specimens to get average value 2020 -11 -30 74/82

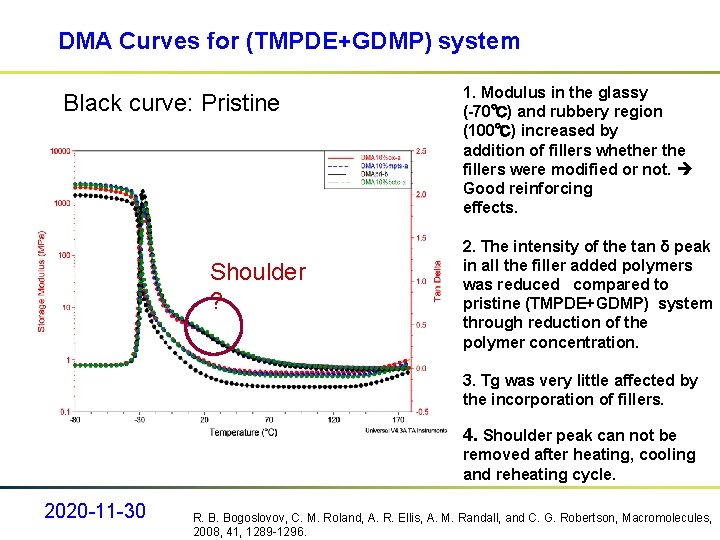
DMA Curves for (TMPDE+GDMP) system Black curve: Pristine Shoulder ? 1. Modulus in the glassy (-70℃) and rubbery region (100℃) increased by addition of fillers whether the fillers were modified or not. Good reinforcing effects. 2. The intensity of the tan δ peak in all the filler added polymers was reduced compared to pristine (TMPDE+GDMP) system through reduction of the polymer concentration. 3. Tg was very little affected by the incorporation of fillers. 4. Shoulder peak can not be removed after heating, cooling and reheating cycle. 2020 -11 -30 R. B. Bogoslovov, C. M. Roland, A. R. Ellis, A. M. Randall, and C. G. Robertson, Macromolecules, 2008, 41, 1289 -1296.

Effects on Tg (-25. 4 ~ -27. 5 ℃ : small effect on Tg means weak interactions between resin molecules and filler surface) 2020 -11 -30 76/82

Glassy Region Storage Modulus Generally increase with filler contents. 2020 -11 -30 77/82

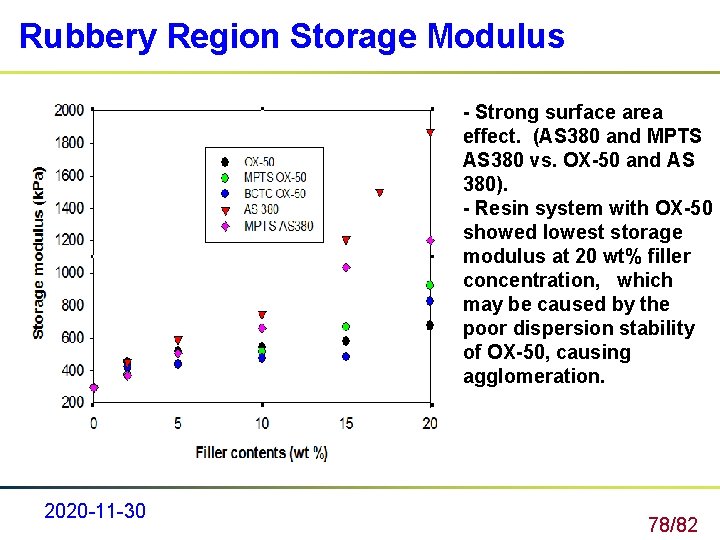
Rubbery Region Storage Modulus - Strong surface area effect. (AS 380 and MPTS AS 380 vs. OX-50 and AS 380). - Resin system with OX-50 showed lowest storage modulus at 20 wt% filler concentration, which may be caused by the poor dispersion stability of OX-50, causing agglomeration. 2020 -11 -30 78/82

Particle Size Effect on Transparency AS 380 (primary particle size: 7 nm) incorporated nanocomposites are more transparent than those of OX-50 (primary particle size: 40 nm) incorporated nanocomposites. (a) 15% OX-50 2020 -11 -30 (b) 15% AS 380

FE-SEM Results for TMPTMP+TATATO To investigate the dispersion state of the silica nano-partilces in the cured resin, cured composites were fractured in liquid nitrogen, and then the morphology of the fractured surface were observed with FE-SEM. 2020 -11 -30 80/82

Results for (TMPDE+GDMP) System 1. The moduli of the TMPDE+GDMP system with silica particles increased. 2. Nanocomposite with smaller particle sizes such as AS 380 & MPTS AS 380 as fillers showed higher modulus than those of OX-50 & MPTS OX-50 incorporated nanocomposites. 2020 -11 -30 81/82

Thank you for your kind attention. 2020 -11 -30 82/82
 Application of relation
Application of relation Expander graphs and their applications
Expander graphs and their applications Application of insulating materials
Application of insulating materials Whose misadventur’d piteous overthrows in modern english
Whose misadventur’d piteous overthrows in modern english Rigid body vs particle
Rigid body vs particle Particle equilibrium in 2d and 3d engineering mechanics
Particle equilibrium in 2d and 3d engineering mechanics Kinetics of a particle force and acceleration
Kinetics of a particle force and acceleration Kinetics of a particle: impulse and momentum
Kinetics of a particle: impulse and momentum Graph the particle's velocity and speed where defined
Graph the particle's velocity and speed where defined Red liquid element
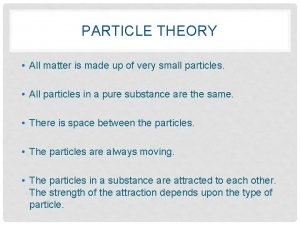
Red liquid element Particle theory of matter grade 7
Particle theory of matter grade 7 Preparation and maintenance of records and reports
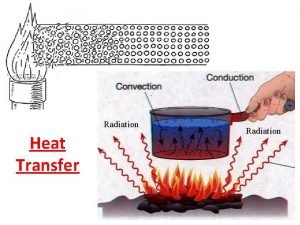
Preparation and maintenance of records and reports Heat and mass transfer fundamentals and applications
Heat and mass transfer fundamentals and applications Image sets
Image sets Particle arrangement of solid
Particle arrangement of solid Wet etch clean and filter
Wet etch clean and filter Wave particle duality questions
Wave particle duality questions Wave-particle duality
Wave-particle duality What is micromeritics
What is micromeritics Alpha particle symbol
Alpha particle symbol Tightly packed in a disorderly manner
Tightly packed in a disorderly manner Particle model of electricity
Particle model of electricity The long-term future of particle accelerators
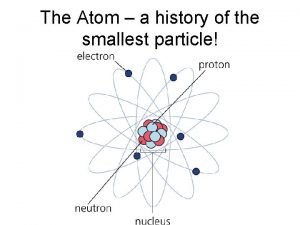
The long-term future of particle accelerators Smallest particle
Smallest particle Pmt particle physics
Pmt particle physics Substance vs mixture
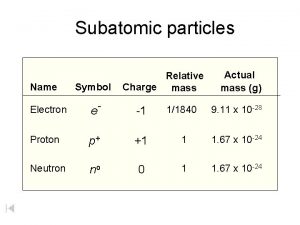
Substance vs mixture Subatomic particles symbol
Subatomic particles symbol Particle theory of matter examples
Particle theory of matter examples Site:slidetodoc.com
Site:slidetodoc.com Soil particle size classification
Soil particle size classification Example of isotones
Example of isotones Rao blackwell particle filter
Rao blackwell particle filter Well behaved wave function
Well behaved wave function Particle model of matter exam questions
Particle model of matter exam questions Chemical vs physical change particle diagram
Chemical vs physical change particle diagram Particulate theory of matter
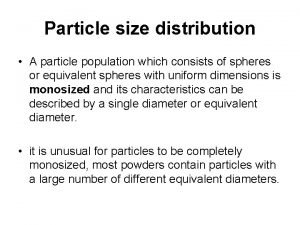
Particulate theory of matter Cumulative particle size distribution curve
Cumulative particle size distribution curve Para view
Para view Matlab particle filter example
Matlab particle filter example How do plasma particles move
How do plasma particles move Louis de broglie wave particle duality
Louis de broglie wave particle duality Smallest particle of an element
Smallest particle of an element Electromagnetic particle inspection
Electromagnetic particle inspection Force on charged particle
Force on charged particle Particle identification
Particle identification Kinematics of a particle
Kinematics of a particle What is a particle
What is a particle Radiation
Radiation Faster than light particle
Faster than light particle Light is a particle evidence
Light is a particle evidence Dynamics of a particle moving in a straight line
Dynamics of a particle moving in a straight line Photon
Photon Sugar dissolving in tea diagram
Sugar dissolving in tea diagram Particle on a ring
Particle on a ring Kinetic energy of a relativistic particle
Kinetic energy of a relativistic particle Rest energy of a proton
Rest energy of a proton Steel interstitial alloy
Steel interstitial alloy Kinetic particle theory of matter
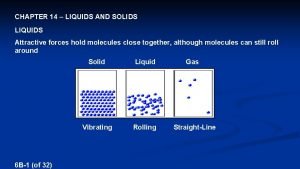
Kinetic particle theory of matter Characterization of solid particles
Characterization of solid particles Blender hair wind
Blender hair wind Symbol for beta particle
Symbol for beta particle The smallest particle an element can be divided into is the
The smallest particle an element can be divided into is the Smallest particle
Smallest particle Atoms are small hard particles
Atoms are small hard particles Erik adli
Erik adli Mark thomson particle physics
Mark thomson particle physics Particle classification diagram
Particle classification diagram Define particle theory of matter
Define particle theory of matter Particle under constant acceleration
Particle under constant acceleration Non cellular particle
Non cellular particle In the box
In the box Particle theory examples
Particle theory examples Physics wordle quark
Physics wordle quark Particle physics
Particle physics Particle diagram of a solid
Particle diagram of a solid Particle bombardment
Particle bombardment Choose the correct particle.
Choose the correct particle. Particle therapy masterclass
Particle therapy masterclass Www.youtube.com
Www.youtube.com Every particle
Every particle Particle physics practice quiz
Particle physics practice quiz Particle filter tutorial
Particle filter tutorial Draw an example of how light travels as a wave
Draw an example of how light travels as a wave