Prenatal Screening for Trisomy 21 Recent Advances and












- Slides: 57

Prenatal Screening for Trisomy 21: Recent Advances and Guidelines Jacob Canick, Ph. D Alpert Medical School of Brown University Women & Infants Hospital Providence, RI, USA Department of Pathology Montefiore Medical Center Bronx, NY March 17, 2011 Women & Infants’ BROWN

Disclosure Information Grant & Research Support: - Beckman Coulter, Inc. , Brea, CA - Beckman Coulter Foundation, Brea, CA - Sequenom, Inc. , San Diego, CA Other: - Patent Holder – u. E 3 in prenatal screening

Prenatal Screening for Trisomy 21: Recent Advances and Guidelines 1. Brief history of prenatal screening 2. How is screening performance measured? 3. Performance of second trimester, first trimester, integrated, and sequential tests for Down syndrome 4. Guidelines: standard of practice in EUROCAT countries and the United States 5. Trisomy 18 and 13 6. Laboratory issues in prenatal screening

Brief history of prenatal screening

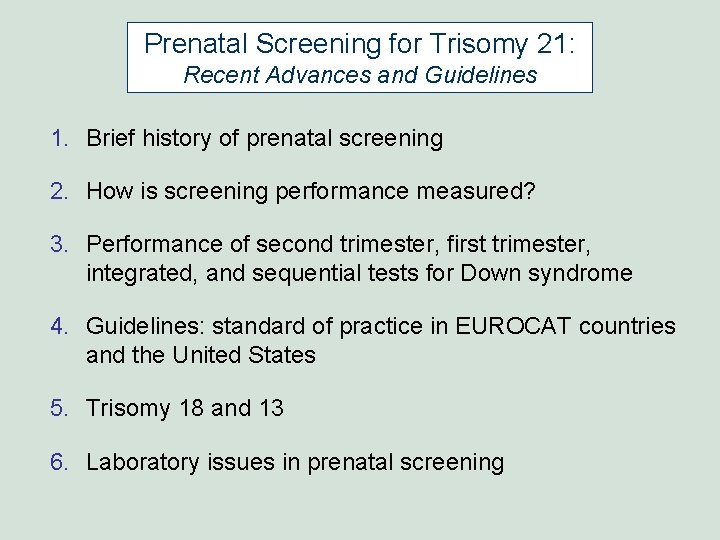
Prenatal Screening History Begins with Prenatal Screening for Open Neural Tube Defects using MSAFP 4 per 1000 births Spina bifida Anencephaly 1970 s AFP in maternal serum elevated in NTDs 1970 s Maternal Serum AFP screening 1980 s acceptance in U. S. (ACOG liability alert) 1980 s primary prevention with folic acid 2000 s move to ultrasound screening? The birth prevalence of spina bifida and anencephaly in England Wales between 1964 and 1975. (N Wald)

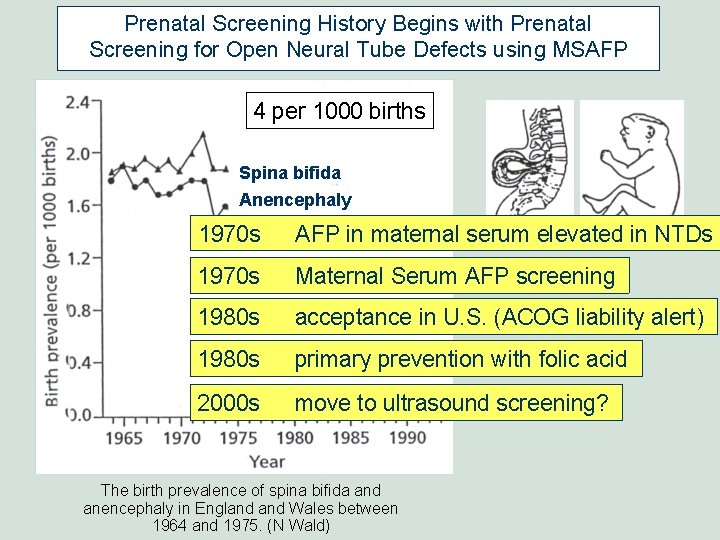
Prenatal Screening History Begins with Prenatal Screening for Open Neural Tube Defects using MSAFP 0. 3 per 1000 births Effect of primary prevention (folic acid) and prenatal screening (MSAFP) The birth prevalence of spina bifida and anencephaly in England Wales between 1964 and 1993. (N Wald)

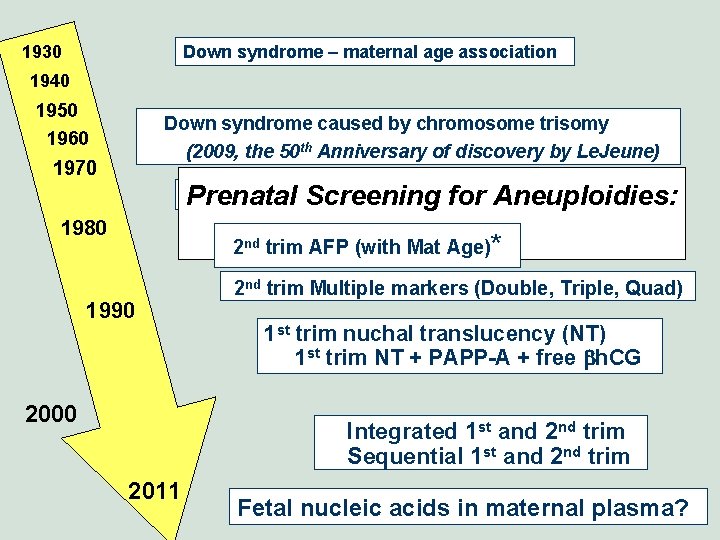
1930 Down syndrome – maternal age association 1940 1950 Down syndrome caused by chromosome trisomy (2009, the 50 th Anniversary of discovery by Le. Jeune) 1960 1970 Screening using maternal age, offer of amnio Prenatal for Aneuploidies: 1980 History 2 nd trim AFP (with Mat Age)* 1990 2000 2 nd trim Multiple markers (Double, Triple, Quad) 1 st trim nuchal translucency (NT) 1 st trim NT + PAPP-A + free bh. CG Integrated 1 st and 2 nd trim Sequential 1 st and 2 nd trim 2011 Fetal nucleic acids in maternal plasma?

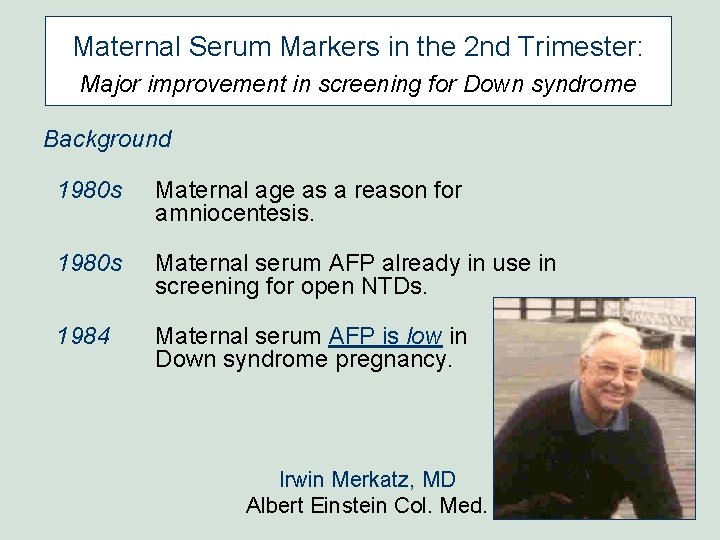
Maternal Serum Markers in the 2 nd Trimester: Major improvement in screening for Down syndrome Background 1980 s Maternal age as a reason for amniocentesis. 1980 s Maternal serum AFP already in use in screening for open NTDs. 1984 Maternal serum AFP is low in Down syndrome pregnancy. Irwin Merkatz, MD Albert Einstein Col. Med.

How is screening performance measured?

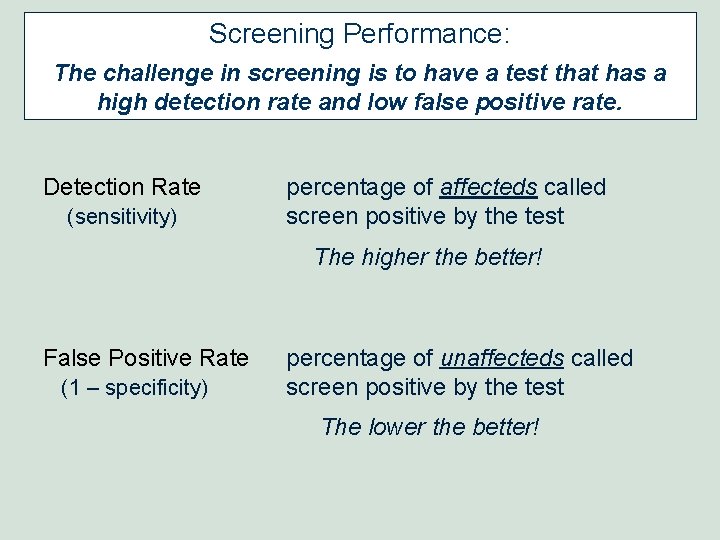
Screening Performance: The challenge in screening is to have a test that has a high detection rate and low false positive rate. Detection Rate (sensitivity) percentage of affecteds called screen positive by the test The higher the better! False Positive Rate (1 – specificity) percentage of unaffecteds called screen positive by the test The lower the better!

Performance of second trimester, first trimester, integrated, and sequential tests for Down syndrome

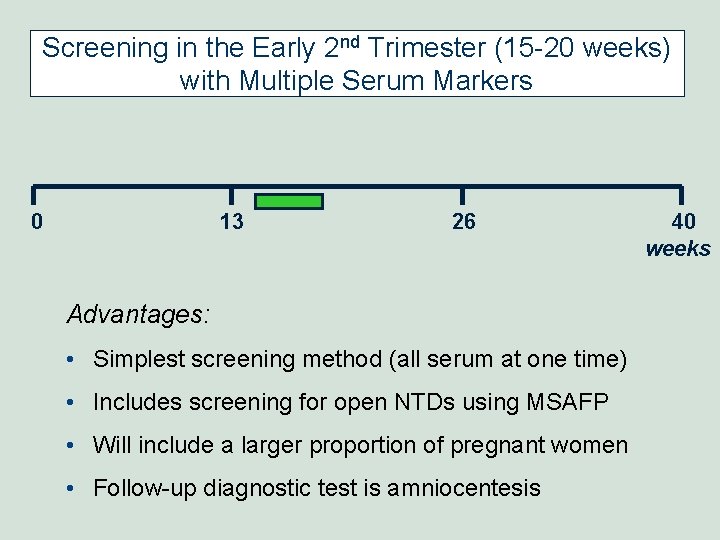
Screening in the Early 2 nd Trimester (15 -20 weeks) with Multiple Serum Markers 0 13 26 Advantages: • Simplest screening method (all serum at one time) • Includes screening for open NTDs using MSAFP • Will include a larger proportion of pregnant women • Follow-up diagnostic test is amniocentesis 40 weeks

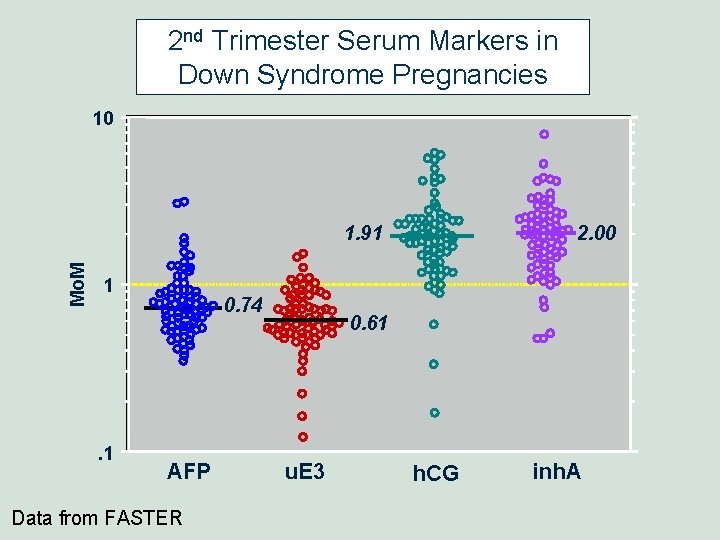
2 nd Trimester Serum Markers in Down Syndrome Pregnancies 10 Mo. M 1. 91 1 . 1 0. 74 AFP Data from FASTER 2. 00 0. 61 u. E 3 h. CG inh. A

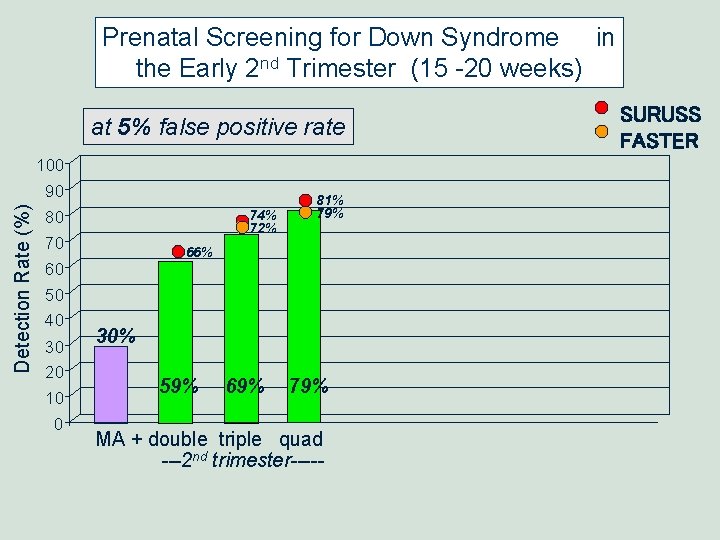
Prenatal Screening for Down Syndrome in the Early 2 nd Trimester (15 -20 weeks) at 5% false positive rate Detection Rate (%) 100 90 80 74% 72% 70 60 50 40 30 20 10 0 81% 79% 66% 30% 59% 69% 79% MA + double triple quad ---2 nd trimester----- SURUSS FASTER

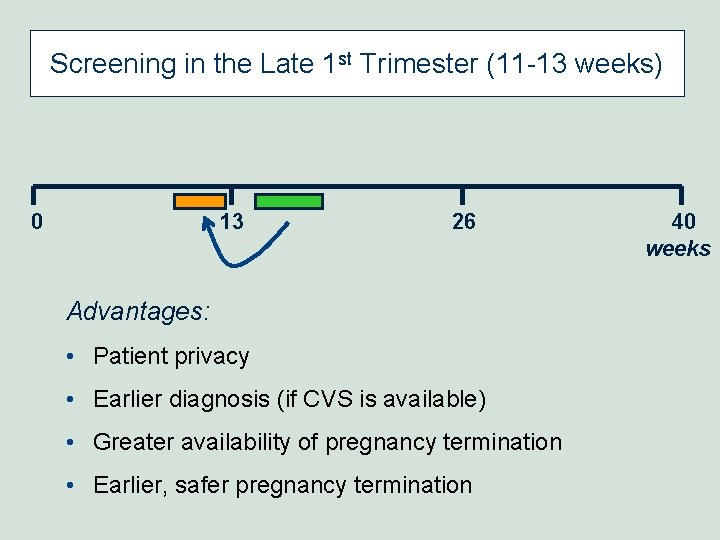
Screening in the Late 1 st Trimester (11 -13 weeks) 0 13 26 Advantages: • Patient privacy • Earlier diagnosis (if CVS is available) • Greater availability of pregnancy termination • Earlier, safer pregnancy termination 40 weeks

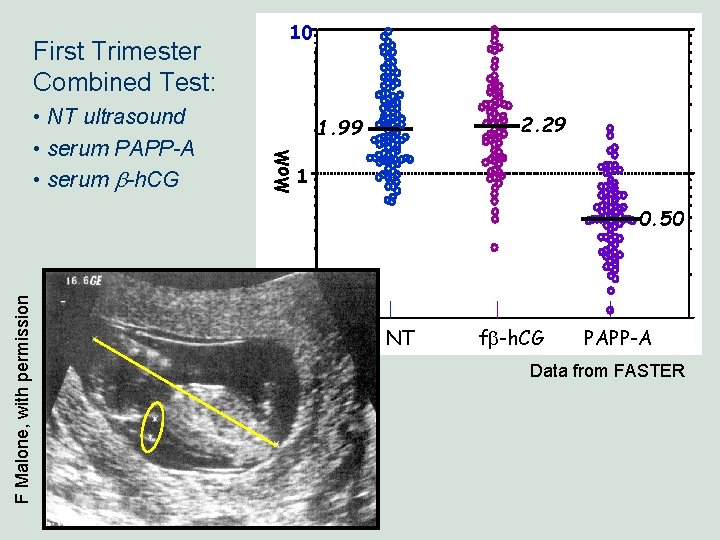
• NT ultrasound • serum PAPP-A • serum b-h. CG 2. 29 1. 99 Mo. M First Trimester Combined Test: 10 1 F Malone, with permission 0. 50 . 1 NT fb-h. CG PAPP-A Data from FASTER

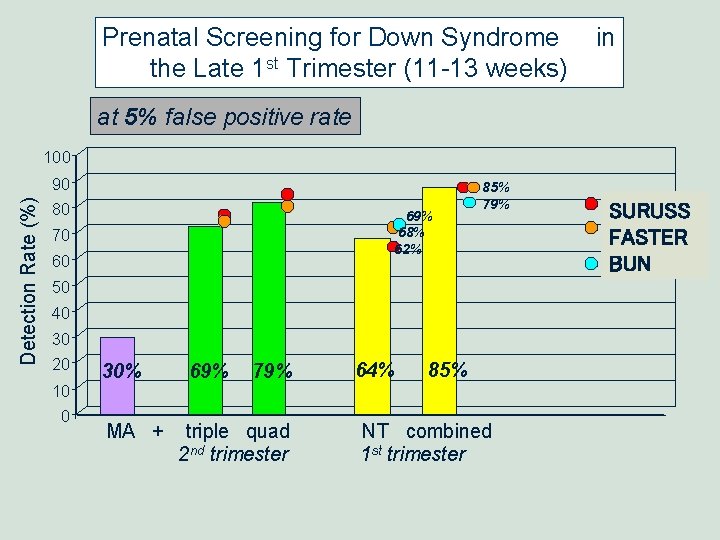
Prenatal Screening for Down Syndrome the Late 1 st Trimester (11 -13 weeks) in at 5% false positive rate Detection Rate (%) 100 90 80 69% 68% 62% 70 60 85% 79% 50 40 30 20 10 0 30% MA + 69% 79% triple quad 2 nd trimester 64% 85% NT combined 1 st trimester SURUSS FASTER BUN

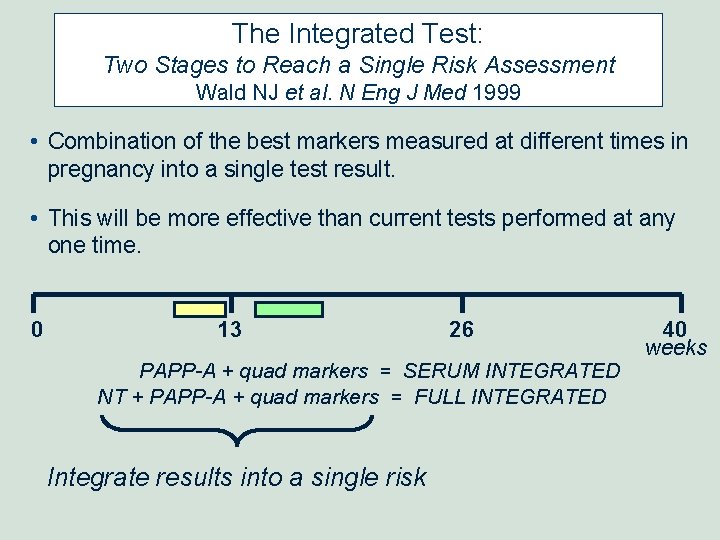
The Integrated Test: Two Stages to Reach a Single Risk Assessment Wald NJ et al. N Eng J Med 1999 • Combination of the best markers measured at different times in pregnancy into a single test result. • This will be more effective than current tests performed at any one time. 0 13 26 PAPP-A + quad markers = SERUM INTEGRATED NT + PAPP-A + quad markers = FULL INTEGRATED Integrate results into a single risk 40 weeks

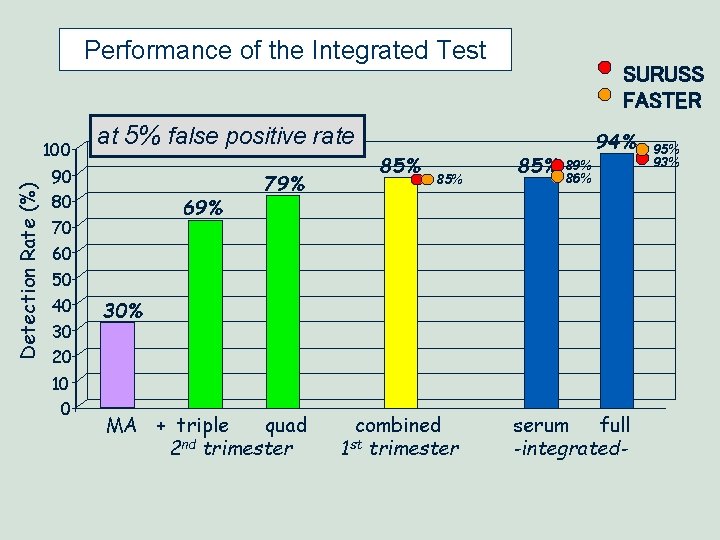
Performance of the Integrated Test Detection Rate (%) 100 at 5% false positive rate 90 80 69% 70 60 50 40 30 20 10 0 SURUSS FASTER 79% 85% 85% 94% 89% 86% 30% MA + triple quad 2 nd trimester combined 1 st trimester serum full -integrated- 95% 93%

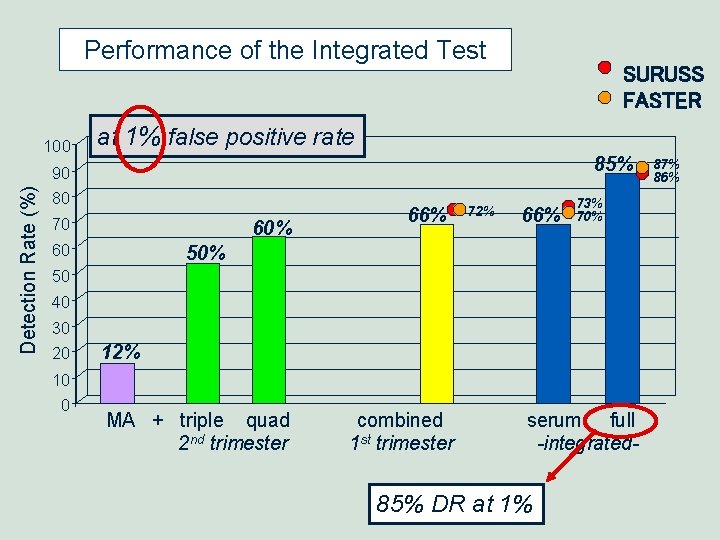
Performance of the Integrated Test Detection Rate (%) 100 SURUSS FASTER at 1% false positive rate 85% 90 80 70 60 60% 66% 72% 66% 73% 70% 50 40 30 20 10 0 12% MA + triple quad 2 nd trimester combined 1 st trimester serum full -integrated- 85% DR at 1% 87% 86%

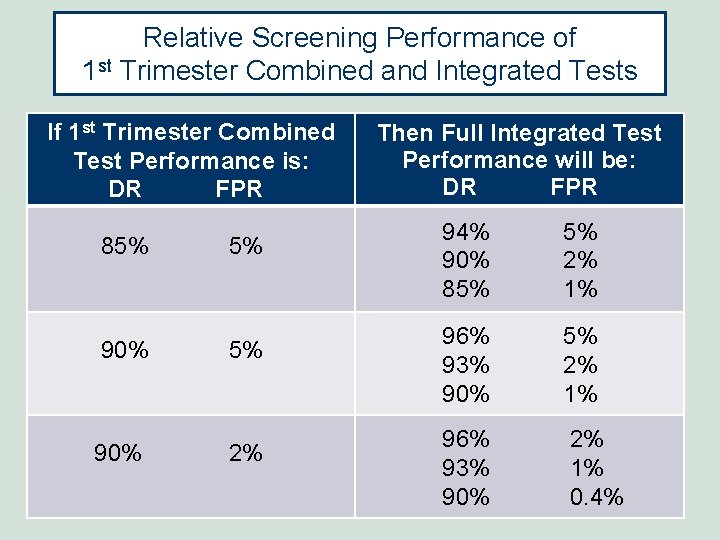
Relative Screening Performance of 1 st Trimester Combined and Integrated Tests If 1 st Trimester Combined Test Performance is: DR FPR 85% 5% 90% 2% Then Full Integrated Test Performance will be: DR FPR 94% 90% 85% 5% 2% 1% 96% 93% 90% 2% 1% 0. 4%

Is there a way to have both: earlier screening and diagnosis – as in the 1 st trimester test and much lower screen positive rate – as in the Integrated Test YES Sequential protocols

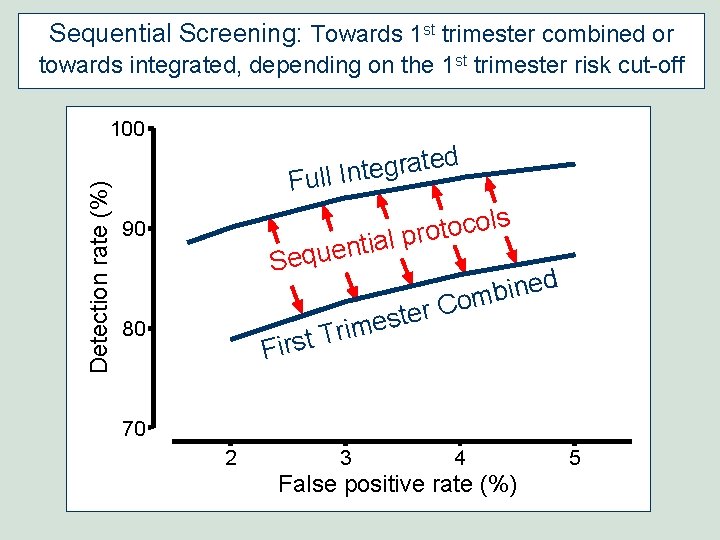
Sequential Screening: Towards 1 st trimester combined or towards integrated, depending on the 1 st trimester risk cut-off Detection rate (%) 100 d e t a r g e Full Int s l o c o t al pro 90 ti n e u q Se ed n i b om C r e t s e m i r T First 80 70 2 3 4 False positive rate (%) 5

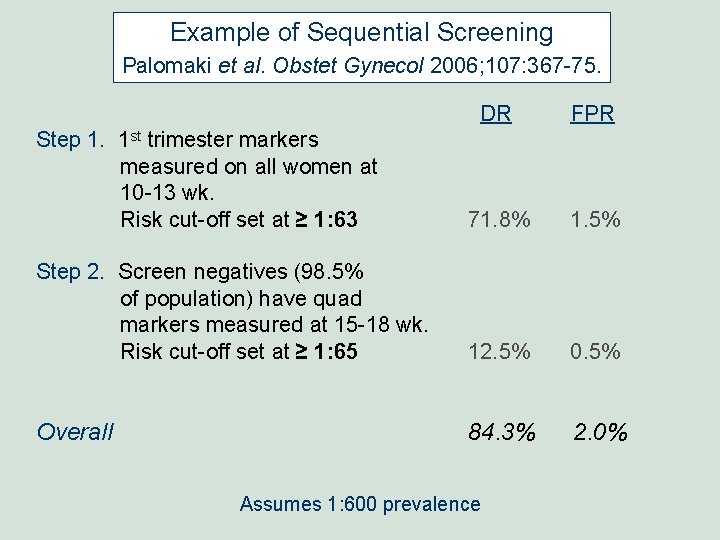
Example of Sequential Screening Palomaki et al. Obstet Gynecol 2006; 107: 367 -75. DR FPR Step 1. 1 st trimester markers measured on all women at 10 -13 wk. Risk cut-off set at ≥ 1: 63 71. 8% 1. 5% Step 2. Screen negatives (98. 5% of population) have quad markers measured at 15 -18 wk. Risk cut-off set at ≥ 1: 65 12. 5% 0. 5% Overall 84. 3% 2. 0% Assumes 1: 600 prevalence

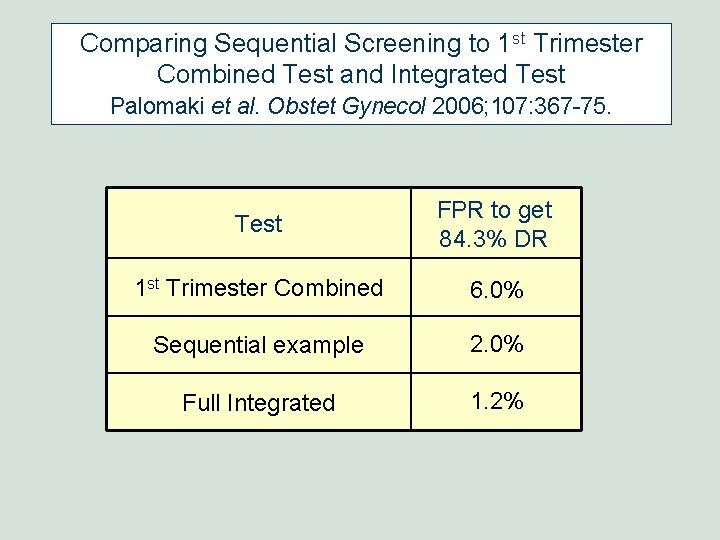
Comparing Sequential Screening to 1 st Trimester Combined Test and Integrated Test Palomaki et al. Obstet Gynecol 2006; 107: 367 -75. Test FPR to get 84. 3% DR 1 st Trimester Combined 6. 0% Sequential example 2. 0% Full Integrated 1. 2%

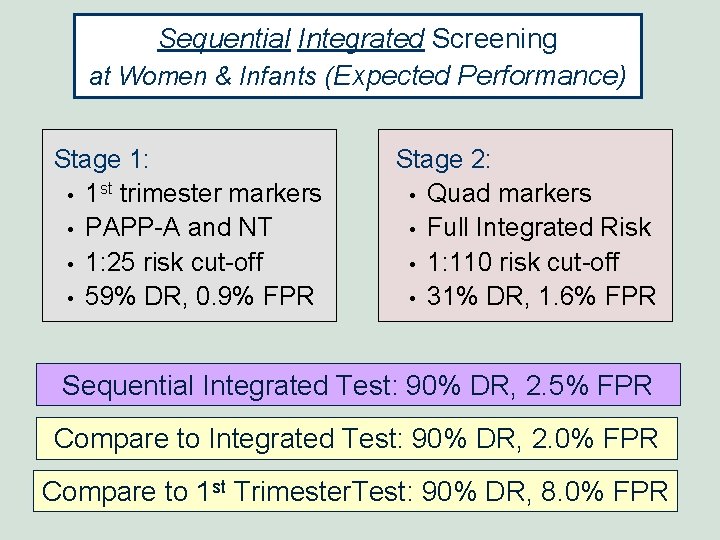
Sequential Integrated Screening at Women & Infants (Expected Performance) Stage 1: • 1 st trimester markers • PAPP-A and NT • 1: 25 risk cut-off • 59% DR, 0. 9% FPR Stage 2: • Quad markers • Full Integrated Risk • 1: 110 risk cut-off • 31% DR, 1. 6% FPR Sequential Integrated Test: 90% DR, 2. 5% FPR Compare to Integrated Test: 90% DR, 2. 0% FPR Compare to 1 st Trimester. Test: 90% DR, 8. 0% FPR

Prenatal Screening Guidelines: Practice Standards in the United States

American College of Obstetricians and Gynecologists (ACOG) January 2007

ACOG 2007 1. “… all women should be offered aneuploidy screening before 20 weeks of gestation, regardless of maternal age. ” 2. “… patients seen early in pregnancy should be offered aneuploidy screening that combines firstand second-trimester testing (integrated or sequential). ” 3. “The screening strategy chosen will depend on availability of CVS and of personnel trained in NT measurement …” ACOG Practice Bulletin, No. 77, January 2007

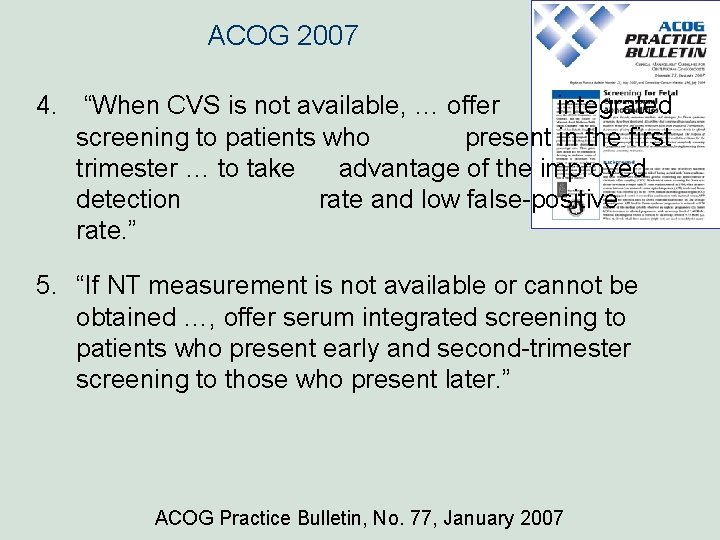
ACOG 2007 4. “When CVS is not available, … offer integrated screening to patients who present in the first trimester … to take advantage of the improved detection rate and low false-positive rate. ” 5. “If NT measurement is not available or cannot be obtained …, offer serum integrated screening to patients who present early and second-trimester screening to those who present later. ” ACOG Practice Bulletin, No. 77, January 2007

Trisomy 18 (Edwards Syndrome) and Trisomy 13 (Patau Syndrome)

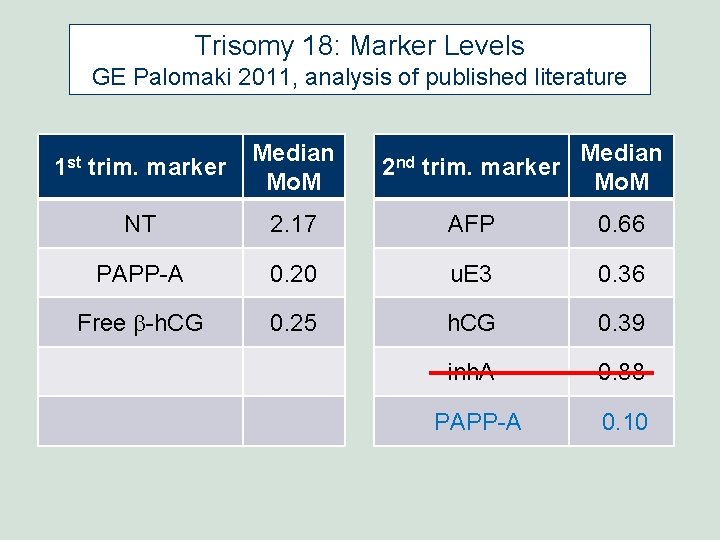
Trisomy 18: Marker Levels GE Palomaki 2011, analysis of published literature 1 st trim. marker Median Mo. M 2 nd trim. marker Median Mo. M NT 2. 17 AFP 0. 66 PAPP-A 0. 20 u. E 3 0. 36 Free b-h. CG 0. 25 h. CG 0. 39 inh. A 0. 88 PAPP-A 0. 10

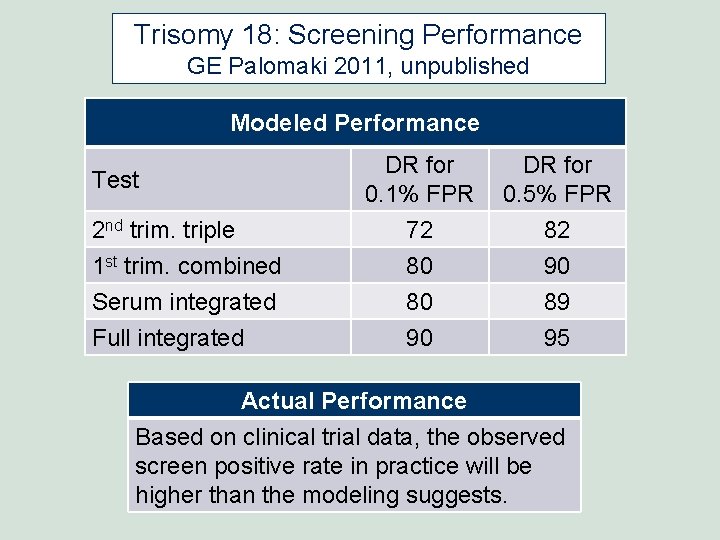
Trisomy 18: Screening Performance GE Palomaki 2011, unpublished Modeled Performance DR for 0. 1% FPR DR for 0. 5% FPR 2 nd trim. triple 72 82 1 st trim. combined Serum integrated Full integrated 80 80 90 90 89 95 Test Actual Performance Based on clinical trial data, the observed screen positive rate in practice will be higher than the modeling suggests.

Trisomy 13 • 2 nd trimester markers are not informative. • 1 st trimester markers are informative. Ø Their pattern is similar to that found in trisomy 18. Ø But, published data on trisomy 13 marker levels are biased and too small to estimate their levels with accuracy. • It is reasonable to assume that the majority of trisomy 13 cases will be identified as part of current trisomy 18 screening protocols that include 1 st trimester markers.

Laboratory Issues in Prenatal Screening • Serum marker assays • Free b-h. CG or total b-h. CG in the 1 st trimester • Screening marker quality assurance • Nuchal Translucency ultrasound and the laboratory

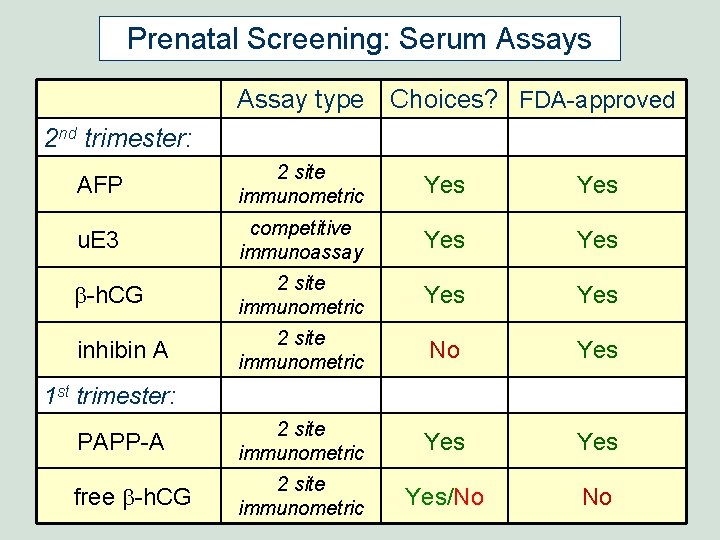
Prenatal Screening: Serum Assays Assay type Choices? FDA-approved 2 nd trimester: AFP 2 site immunometric Yes u. E 3 competitive immunoassay Yes b-h. CG 2 site immunometric Yes inhibin A 2 site immunometric No Yes PAPP-A 2 site immunometric Yes free b-h. CG 2 site immunometric Yes/No No 1 st trimester:

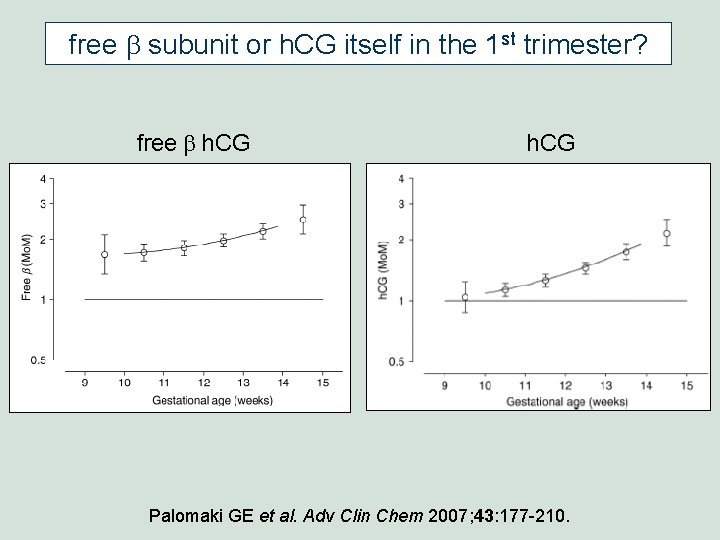
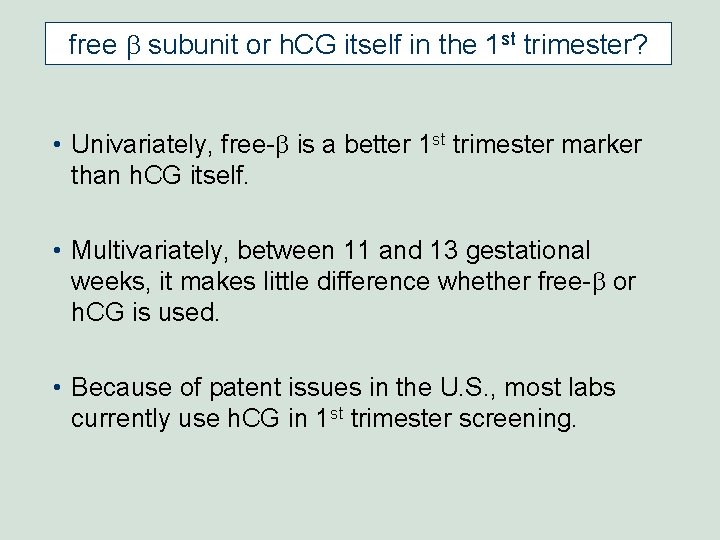
free b subunit or h. CG itself in the 1 st trimester? free b h. CG Palomaki GE et al. Adv Clin Chem 2007; 43: 177 -210.

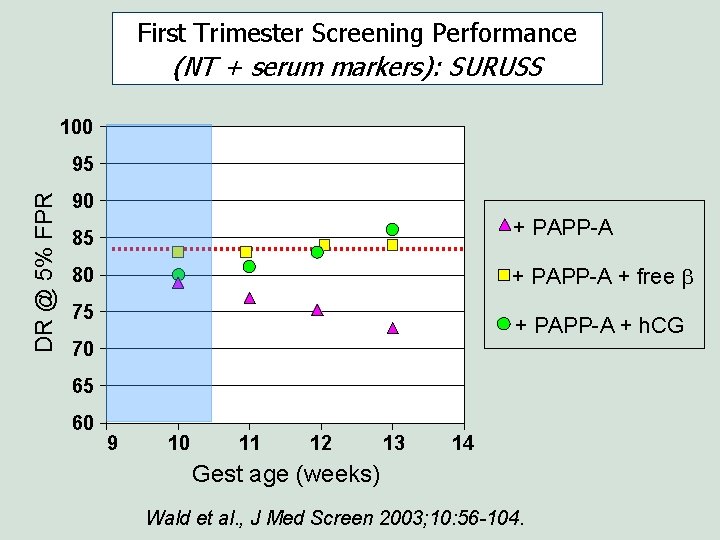
First Trimester Screening Performance (NT + serum markers): SURUSS 100 DR @ 5% FPR 95 90 + PAPP-A 85 + PAPP-A + free b 80 75 + PAPP-A + h. CG 70 65 60 9 10 11 12 13 14 Gest age (weeks) Wald et al. , J Med Screen 2003; 10: 56 -104.

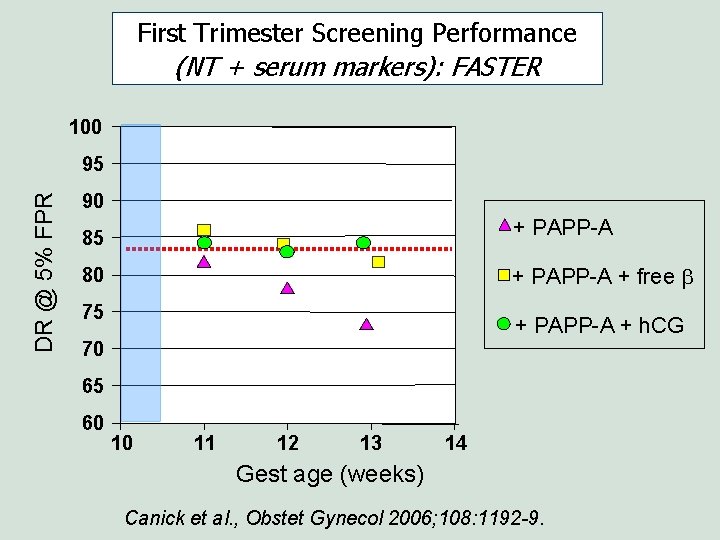
First Trimester Screening Performance (NT + serum markers): FASTER 100 DR @ 5% FPR 95 90 + PAPP-A 85 + PAPP-A + free b 80 75 + PAPP-A + h. CG 70 65 60 10 11 12 13 14 Gest age (weeks) Canick et al. , Obstet Gynecol 2006; 108: 1192 -9.

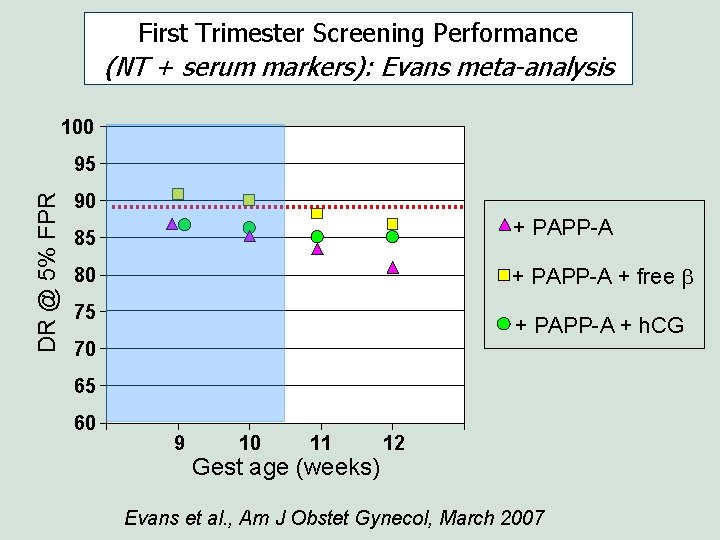
First Trimester Screening Performance (NT + serum markers): Evans meta-analysis 100 DR @ 5% FPR 95 90 + PAPP-A 85 + PAPP-A + free b 80 75 + PAPP-A + h. CG 70 65 60 9 10 11 Gest age (weeks) 12 Evans et al. , Am J Obstet Gynecol, March 2007

free b subunit or h. CG itself in the 1 st trimester? • Univariately, free-b is a better 1 st trimester marker than h. CG itself. • Multivariately, between 11 and 13 gestational weeks, it makes little difference whether free-b or h. CG is used. • Because of patent issues in the U. S. , most labs currently use h. CG in 1 st trimester screening.

Prenatal Screening Quality Assurance Applying Serum Marker Quality Assurance Measures to Nuchal Translucency (NT)

Prenatal Screening Markers Quality monitoring Marker parameters that are monitored: Rate of change with increasing gestation may go up or down at a constant rate Median calculated Mo. M values should be stable at 1. 0 SD of the distribution calculated SD of the log Mo. M values expected to remain steady

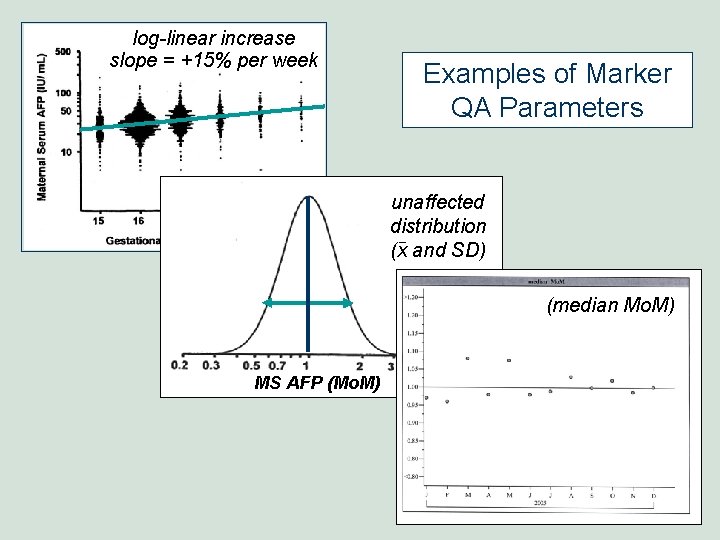
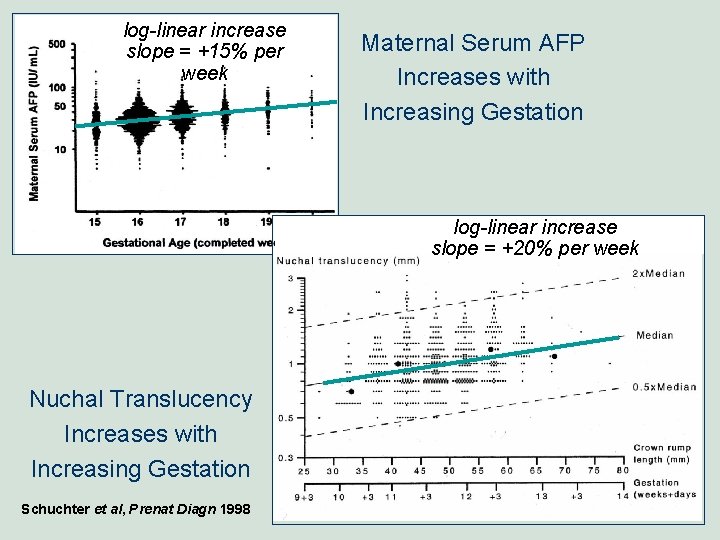
log-linear increase slope = +15% per week Examples of Marker QA Parameters unaffected distribution (x and SD) (median Mo. M) MS AFP (Mo. M)

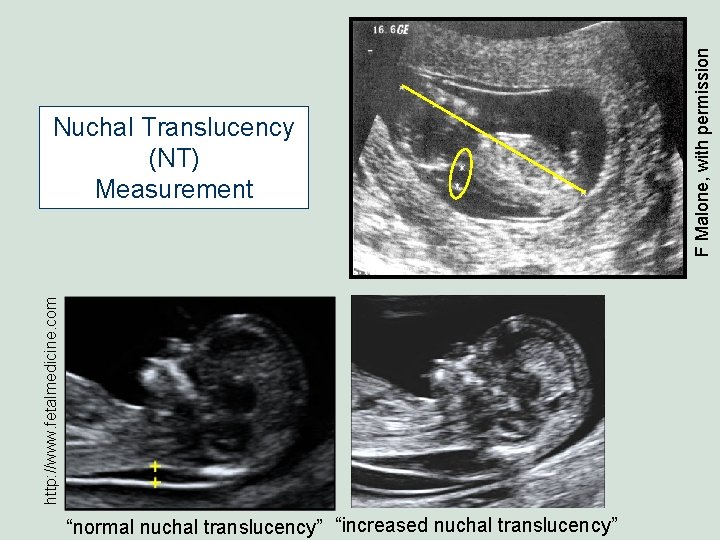
http: //www. fetalmedicine. com “normal nuchal translucency” “increased nuchal translucency” F Malone, with permission Nuchal Translucency (NT) Measurement

1 st Trimester Nuchal Translucency (NT) Quality monitoring NT parameters that are monitored: Rate of increase with CRL log-linear over 10, 3 - 13, 6 weeks should go up by ~ 20% per week Median calculated Mo. M values should be stable at 1. 0 Mo. M SD of the distribution calculated SD of the log Mo. M values expected to be about 0. 1

log-linear increase slope = +15% per week Maternal Serum AFP Increases with Increasing Gestation log-linear increase slope = +20% per week Nuchal Translucency Increases with Increasing Gestation Schuchter et al, Prenat Diagn 1998

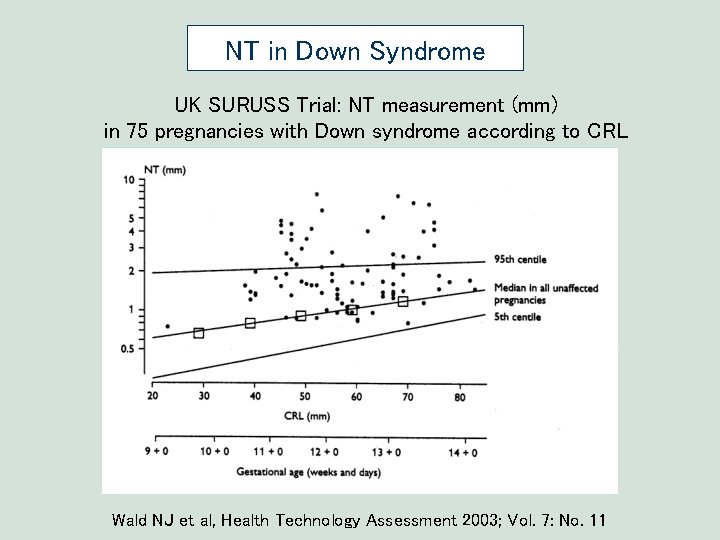
NT in Down Syndrome UK SURUSS Trial: NT measurement (mm) in 75 pregnancies with Down syndrome according to CRL Wald NJ et al, Health Technology Assessment 2003; Vol. 7: No. 11

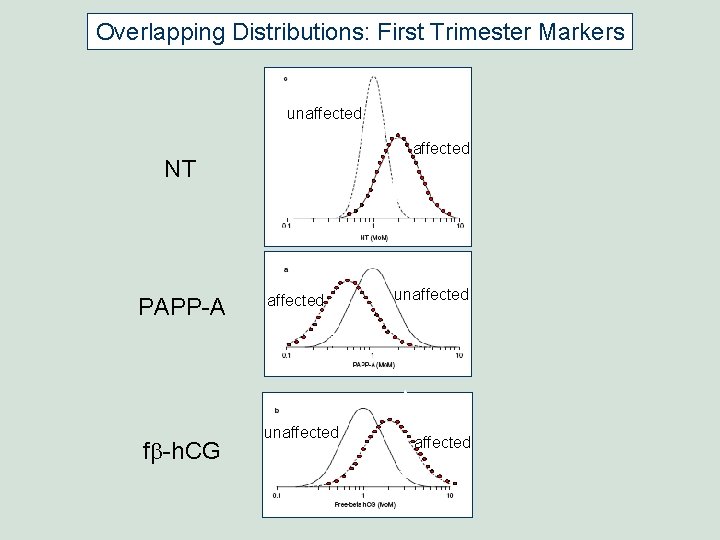
Overlapping Distributions: First Trimester Markers unaffected NT PAPP-A fb-h. CG affected unaffected

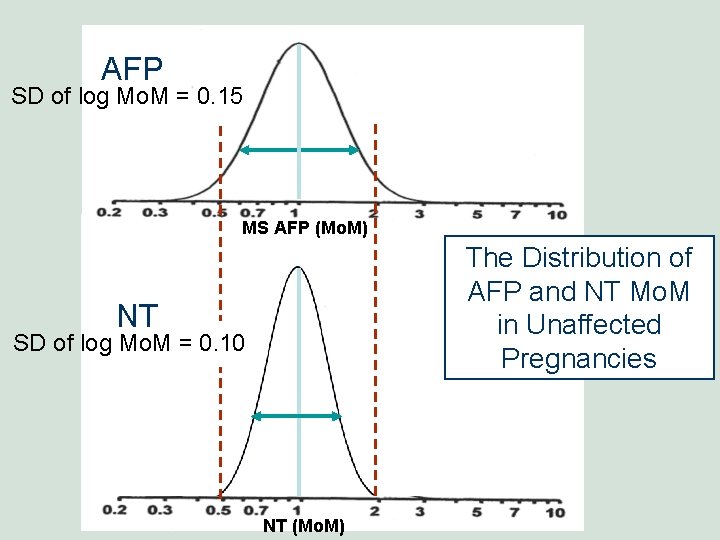
AFP SD of log Mo. M = 0. 15 MS AFP (Mo. M) The Distribution of AFP and NT Mo. M in Unaffected Pregnancies NT SD of log Mo. M = 0. 10 NT (Mo. M)

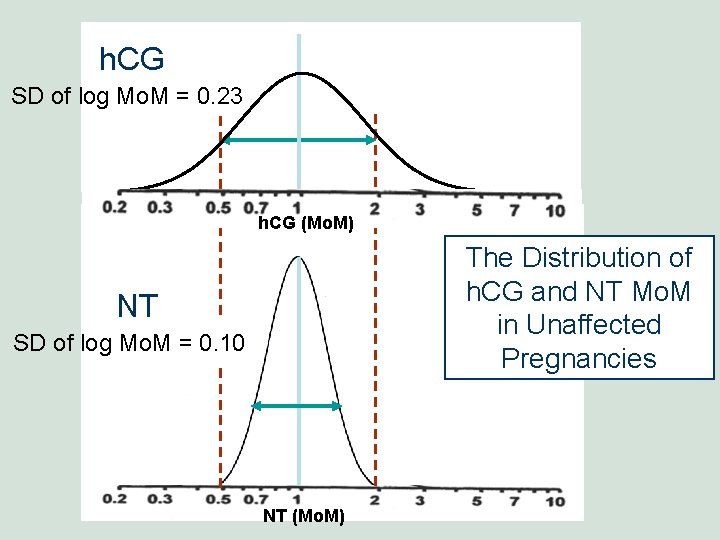
h. CG SD of log Mo. M = 0. 23 0. 2 MS AFP(Mo. M) h. CG 10 The Distribution of h. CG and NT Mo. M in Unaffected Pregnancies NT SD of log Mo. M = 0. 10 NT (Mo. M)

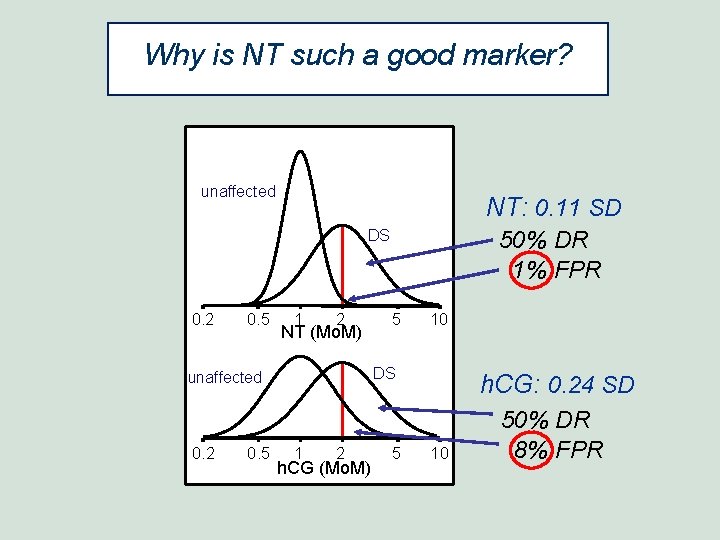
Why is NT such a good marker? unaffected NT: 0. 11 SD DS 0. 2 0. 5 1 2 NT (Mo. M) 0. 5 5 10 DS unaffected 0. 2 50% DR 1% FPR 1 2 h. CG (Mo. M) 5 h. CG: 0. 24 SD 10 50% DR 8% FPR

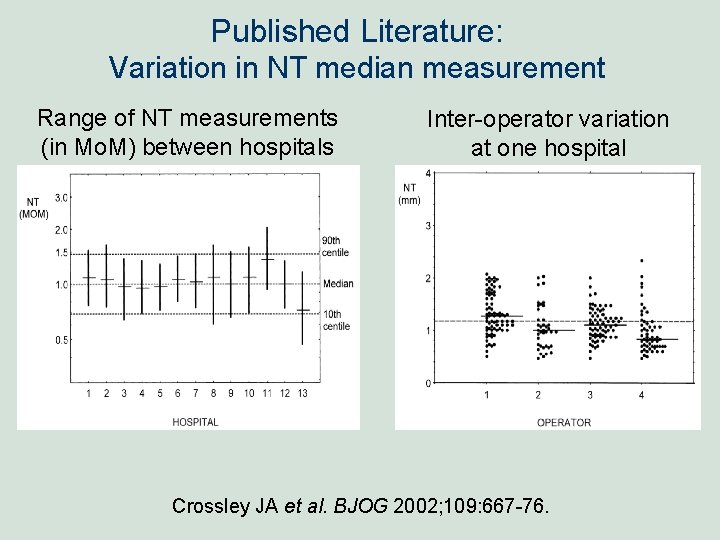
Published Literature: Variation in NT median measurement Range of NT measurements (in Mo. M) between hospitals Inter-operator variation at one hospital Crossley JA et al. BJOG 2002; 109: 667 -76.

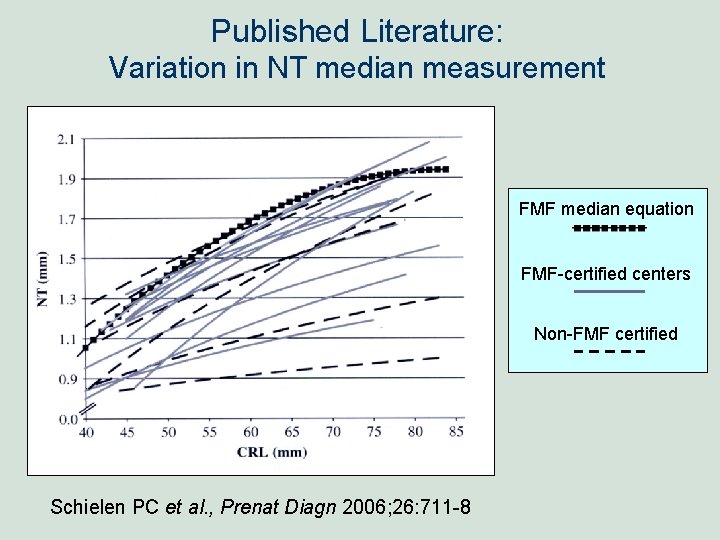
Published Literature: Variation in NT median measurement FMF median equation FMF-certified centers Non-FMF certified Schielen PC et al. , Prenat Diagn 2006; 26: 711 -8

Palomaki GE et al. Genet Med 2008; 10(2): 131 -138 “Laboratories should routinely monitor the quality of nuchal translucency measurements… When possible, instituting sonographer-specific medians and providing individualized feedback about performance and numbers of women tested offer the potential to yield more consistent and improved performance. ”

NT monitoring: when to make changes § Use objective criteria as guide § Partially subjective process § Look for trends § Sample volume must be considered § What to do with very small volume sonographers? § Sonographer feedback has been minimally useful

-----Women & Infants Hospital - Brown University----- Geralyn Lambert-Messerlian Jim Haddow Primary collaborators over many years Glenn Palomaki George Knight University of London Nick Wald