PreLicensure Clinical Trials How Big Is Big Enough

Pre-Licensure Clinical Trials: How Big Is Big Enough? Paul A. Offit, MD Children’s Hospital of Philadelphia Univ. of Pennsylvania School of Medicine

Development of modern vaccines Several factors influence the size of prelicensure clinical trials. u Best understood by examining the decade in which the vaccine was made. u

Pre-licensure clinical trials Decade Vaccine Trial size 1950 s Polio, inactivated 1, 800, 000 Francis, T. Evaluation of the 1954 Field Trial of Poliomyelitis Vaccine, April 1957

Polio vaccine, inactivated 420, 000 given vaccine; 200, 000 injected with placebo; 1, 200, 000 observed uninoculated controls. u 34 unvaccinated and 2 vaccinated children developed severe paralysis. u 16 unvaccinated children and 0 vaccinated children died from polio. u
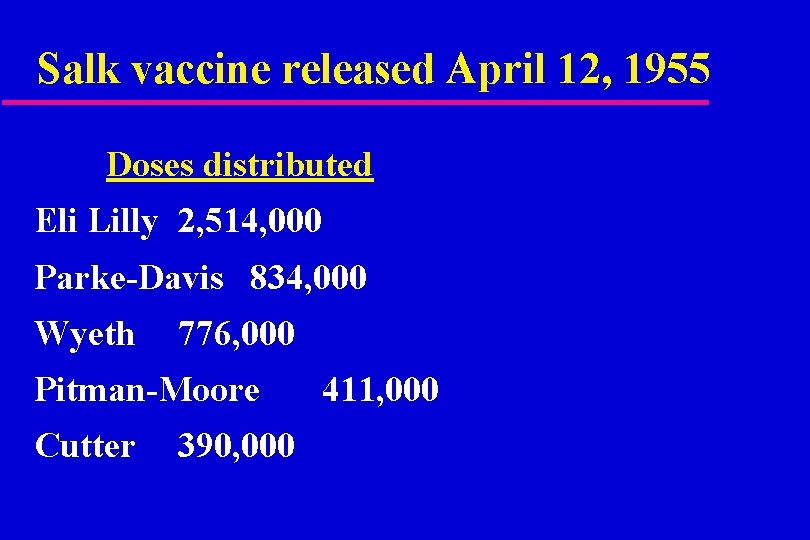
Salk vaccine released April 12, 1955 Doses distributed Eli Lilly 2, 514, 000 Parke-Davis 834, 000 Wyeth 776, 000 Pitman-Moore Cutter 390, 000 411, 000

The Cutter Incident u 120, 000 children inoculated with live poliovirus. u 100, 000 family and community contacts were also infected. Offit, PA. The Cutter Incident, Yale University Press, 2005

The Cutter Incident u At least 70, 000 people developed abortive polio. u 164 people were permanently paralyzed. u 10 people were killed. Birth of vaccine regulation in U. S. Vaccines had to be tested pre-licensure. u

Pre-licensure clinical trials Decade Vaccine Trial size 1950 s Polio, live attenuated -

Polio vaccine, live attenuated 90 million people inoculated in USSR; trial evaluated by Dorothy Horstmann. u Between 1961 and 1964 about 100 million doses administered in U. S. u VAPP occurred in 1 per 750, 000 first doses and 1 per 2. 5 M total doses. Now, US data required. u Paul, JR. A History of Poliomyelitis. Yale University Press, 1971

Pre-licensure clinical trials Decade Vaccine Trial size 1960 s Measles 900 Mumps 1, 340 Rubella 5, 000
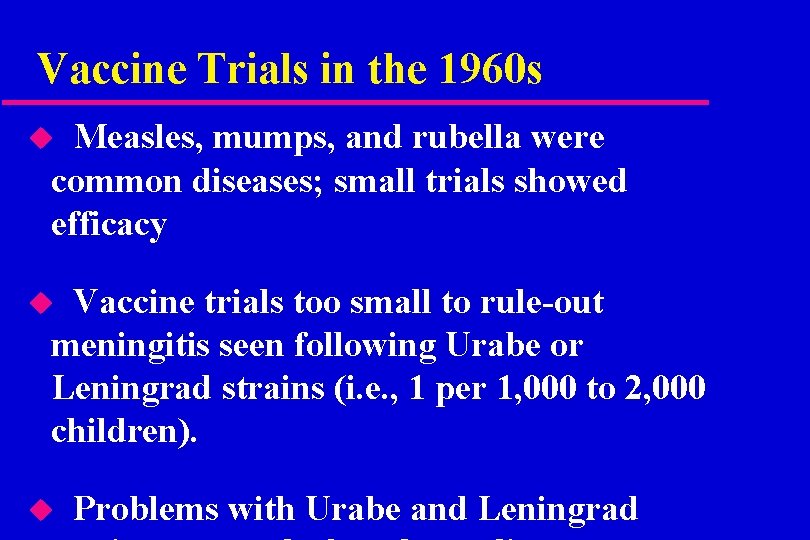
Vaccine Trials in the 1960 s Measles, mumps, and rubella were common diseases; small trials showed efficacy u Vaccine trials too small to rule-out meningitis seen following Urabe or Leningrad strains (i. e. , 1 per 1, 000 to 2, 000 children). u u Problems with Urabe and Leningrad

“I never breathe a sigh of relief until the first 3 million doses are out there. ” Maurice R. Hilleman

Pre-licensure clinical trials Decade Vaccine Trial size 1970 s Rubella 8, 000
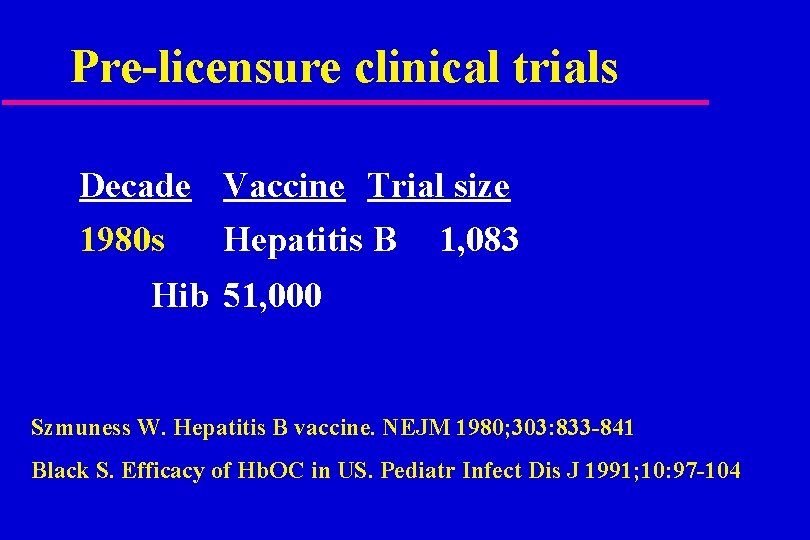
Pre-licensure clinical trials Decade Vaccine Trial size 1980 s Hepatitis B 1, 083 Hib 51, 000 Szmuness W. Hepatitis B vaccine. NEJM 1980; 303: 833 -841 Black S. Efficacy of Hb. OC in US. Pediatr Infect Dis J 1991; 10: 97 -104
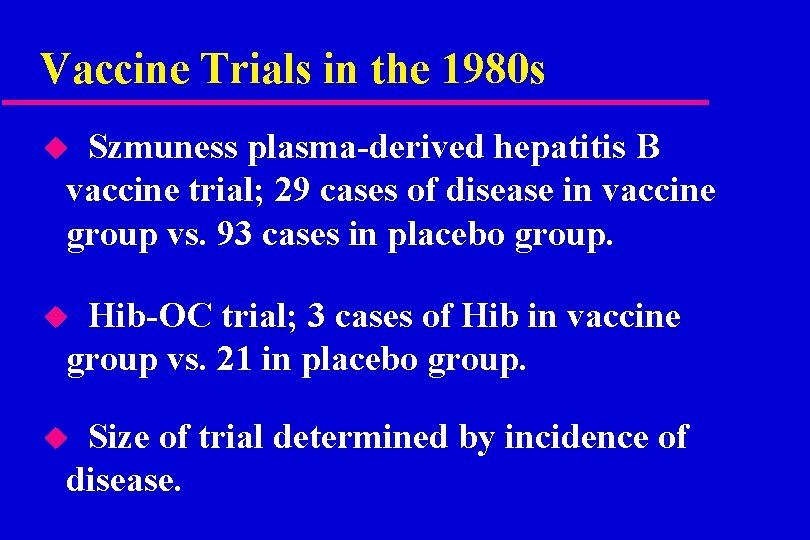
Vaccine Trials in the 1980 s Szmuness plasma-derived hepatitis B vaccine trial; 29 cases of disease in vaccine group vs. 93 cases in placebo group. u Hib-OC trial; 3 cases of Hib in vaccine group vs. 21 in placebo group. u Size of trial determined by incidence of disease. u
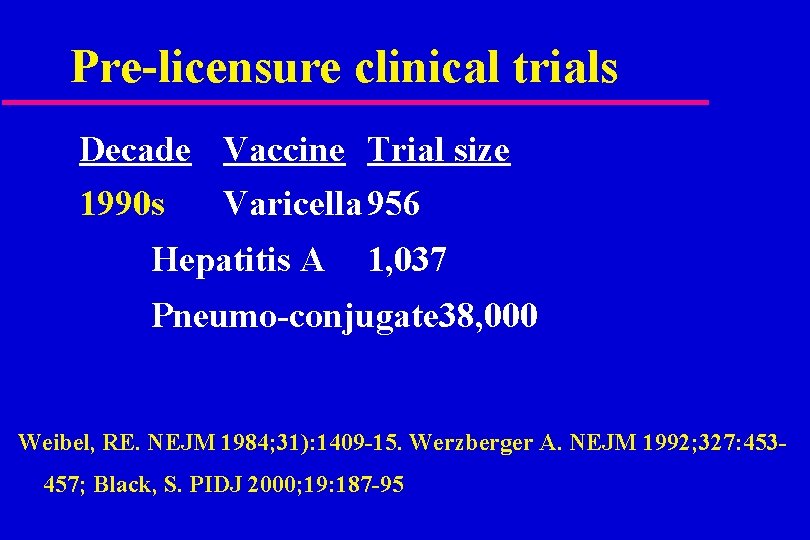
Pre-licensure clinical trials Decade Vaccine Trial size 1990 s Varicella 956 Hepatitis A 1, 037 Pneumo-conjugate 38, 000 Weibel, RE. NEJM 1984; 31): 1409 -15. Werzberger A. NEJM 1992; 327: 453457; Black, S. PIDJ 2000; 19: 187 -95

Vaccines in the 1990 s Hepatitis A trial in Kiryat Joel; 0 cases in the vaccine group and 25 in placebo group. u Pneumococcal conjugate trial in California: 0 cases of invasive disease in vaccine group and 17 in control group. u Size of trial determined by incidence of disease. u

Pre-licensure clinical trials Decade Vaccine Trial size 1990 s Rotavirus 6, 100 Clark HF, et al. Rotavirus vaccines in Plotkin, Orenstein, in Vaccines

Rotavirus vaccine (Rota. Shield) Post-licensure, after vaccine given to about 1 million children, found to cause intussusception in 1 per 10, 000 vaccinees. u Incidence of intussusception post. Rota. Shield was too low to be observed in pre -licensure trial. u
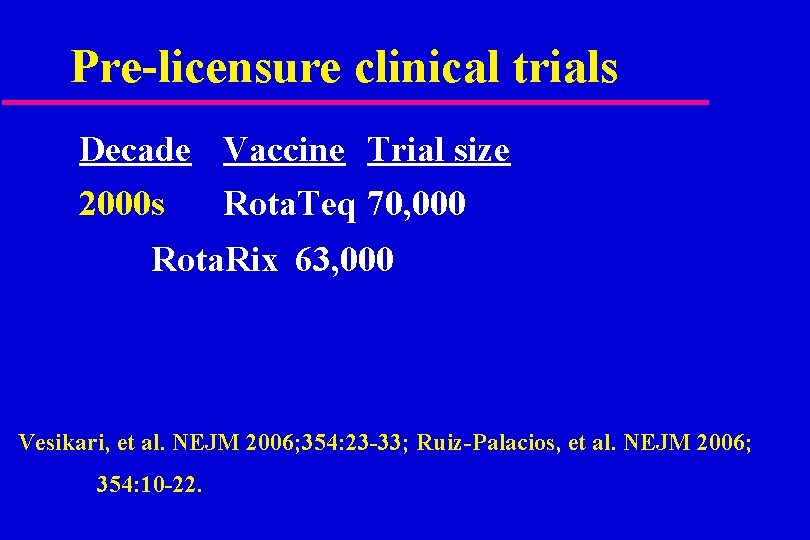
Pre-licensure clinical trials Decade Vaccine Trial size 2000 s Rota. Teq 70, 000 Rota. Rix 63, 000 Vesikari, et al. NEJM 2006; 354: 23 -33; Ruiz-Palacios, et al. NEJM 2006; 354: 10 -22.
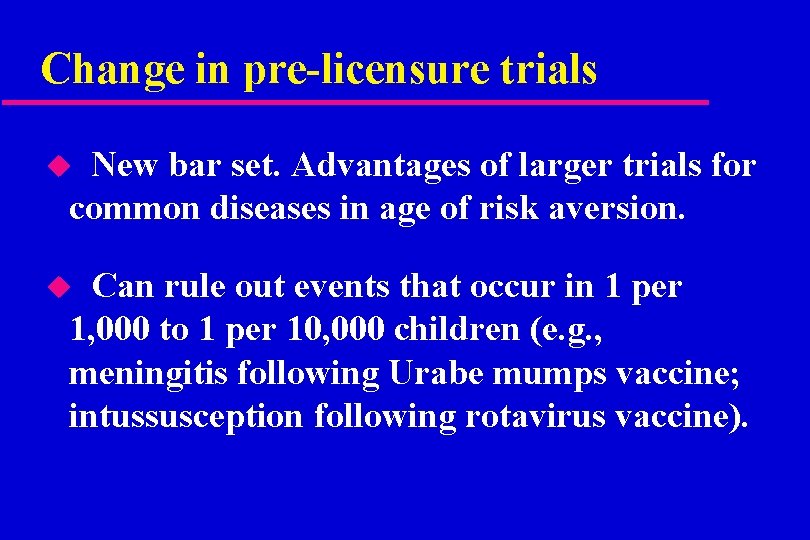
Change in pre-licensure trials New bar set. Advantages of larger trials for common diseases in age of risk aversion. u Can rule out events that occur in 1 per 1, 000 to 1 per 10, 000 children (e. g. , meningitis following Urabe mumps vaccine; intussusception following rotavirus vaccine). u

Change in pre-licensure trials Very rare adverse events will never be ruled out pre-licensure: GBS following Menactra or swine flu; paralysis following OPV; thrombocytopenia following measles vaccine. Hence, VAERS and VSD. u Hilleman’s observation forty years ago remains true. Cost of trial of 3 million children is prohibitive. u

Summary Recent experiences with Rota. Shield vaccine have probably permanently raised the bar to perform larger pre-licensure safety trials. u Although cost is great, there advantages to larger trials in age of risk aversion and aggressive litigation. However, this will also increase the cost of vaccines. u
- Slides: 23