Preimplantation Genetic Diagnosis PGD in Medicine Dr Hazem












- Slides: 46

Preimplantation Genetic Diagnosis (PGD) in Medicine Dr. Hazem Al-Rumaih – FRCOG , MD Consultant OBGYN & reproductive Medicine

Introduction • The past 100 yrs have given birth to the most profound changes in society, medicine & technology the world have ever witnessed. • Genetics is one such field that have enjoyed a meteoric rise during this time.

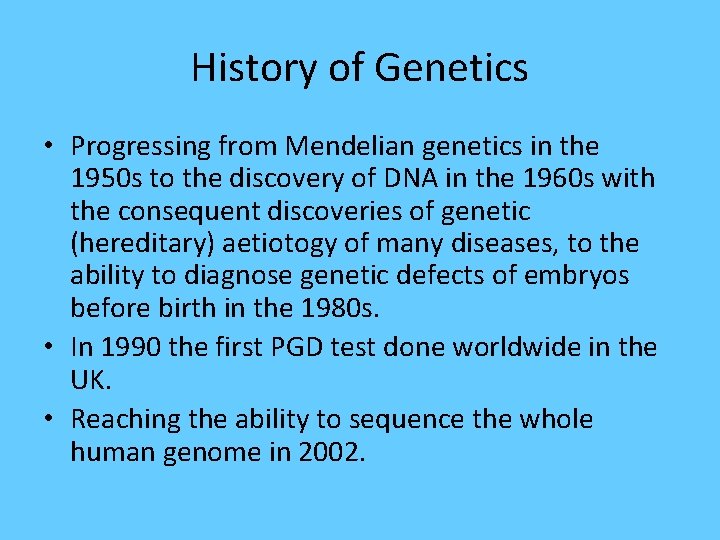
History of Genetics • Progressing from Mendelian genetics in the 1950 s to the discovery of DNA in the 1960 s with the consequent discoveries of genetic (hereditary) aetiotogy of many diseases, to the ability to diagnose genetic defects of embryos before birth in the 1980 s. • In 1990 the first PGD test done worldwide in the UK. • Reaching the ability to sequence the whole human genome in 2002.

The Consequences • This magnificent development in genetics have shifted medicine completely from only diagnosing to preventing hereditary disorders.

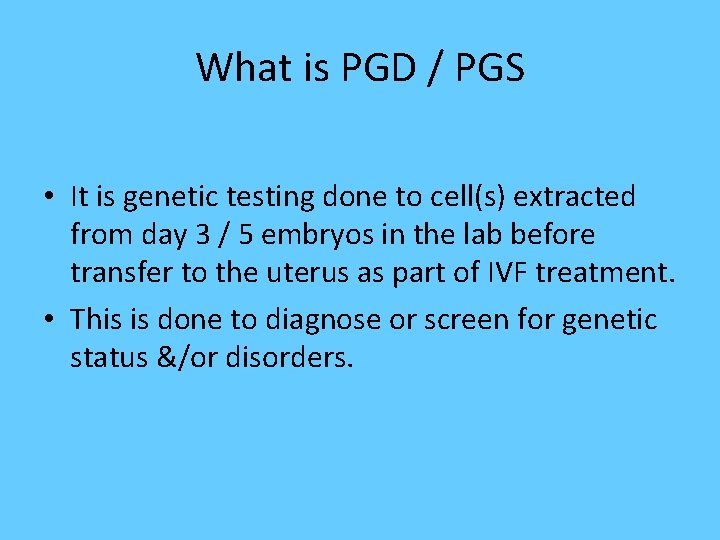
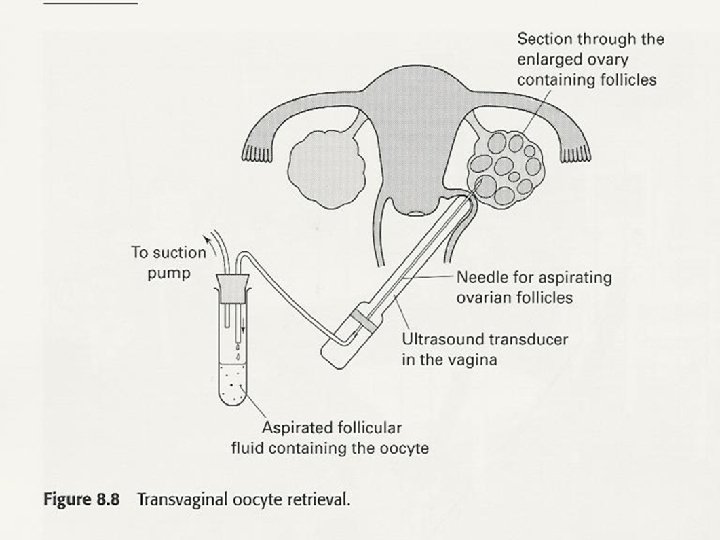
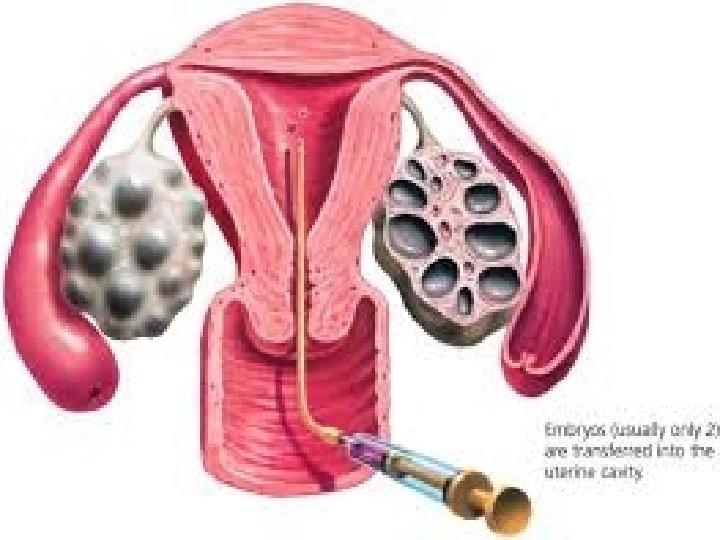
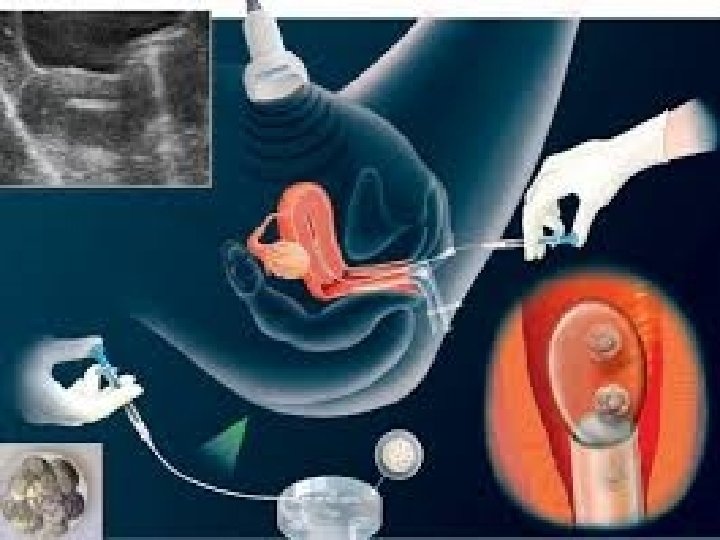
What is PGD / PGS • It is genetic testing done to cell(s) extracted from day 3 / 5 embryos in the lab before transfer to the uterus as part of IVF treatment. • This is done to diagnose or screen for genetic status &/or disorders.



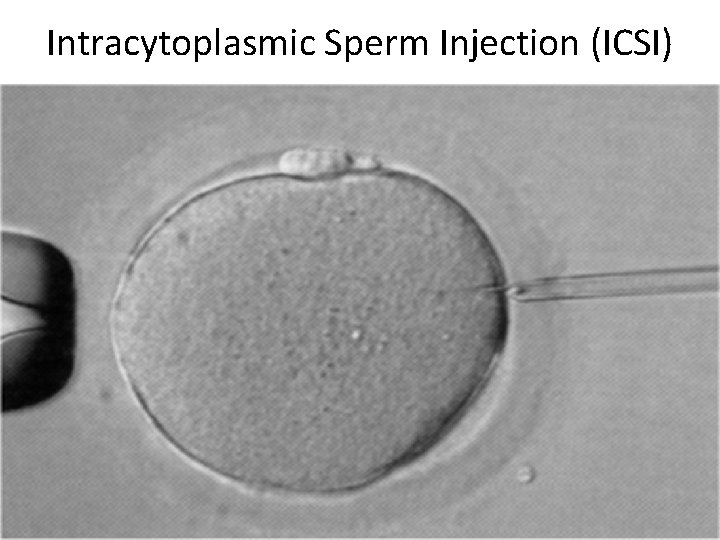
Intracytoplasmic Sperm Injection (ICSI)

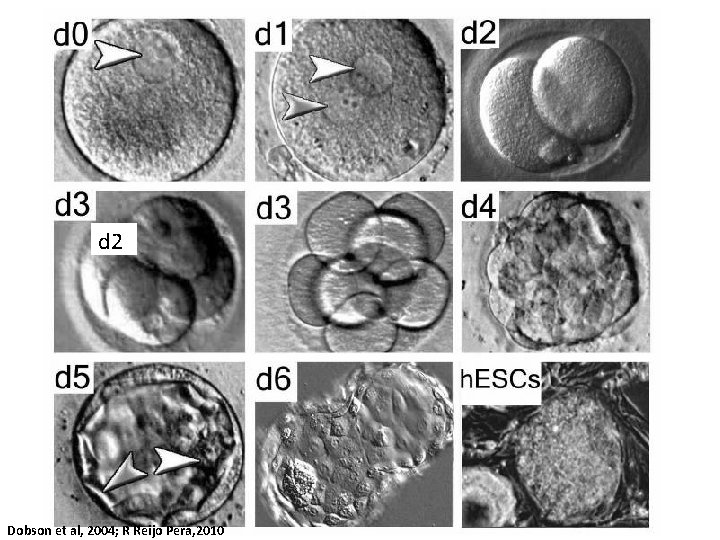
d 2 Dobson et al, 2004; R Reijo Pera, 2010

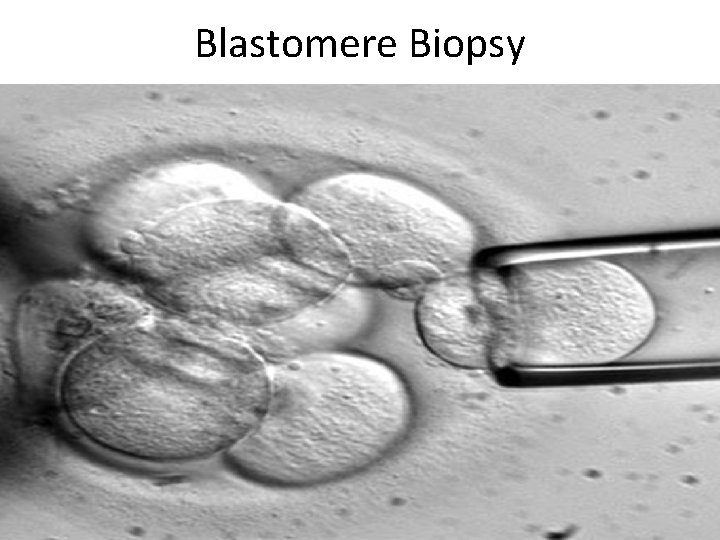
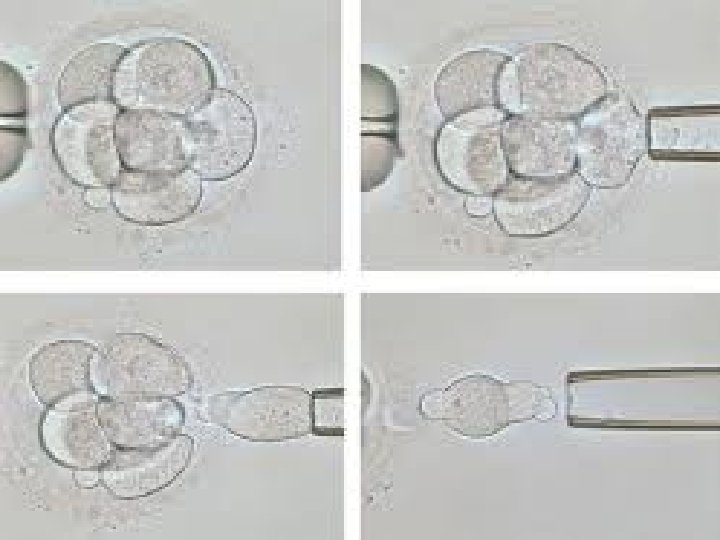
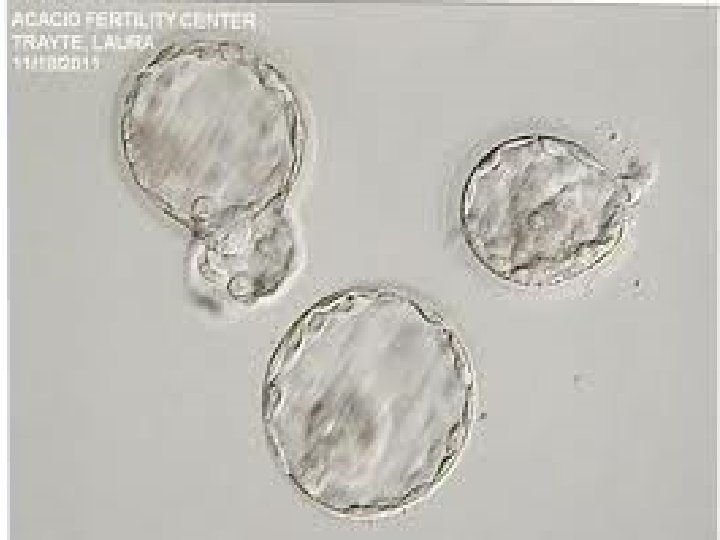
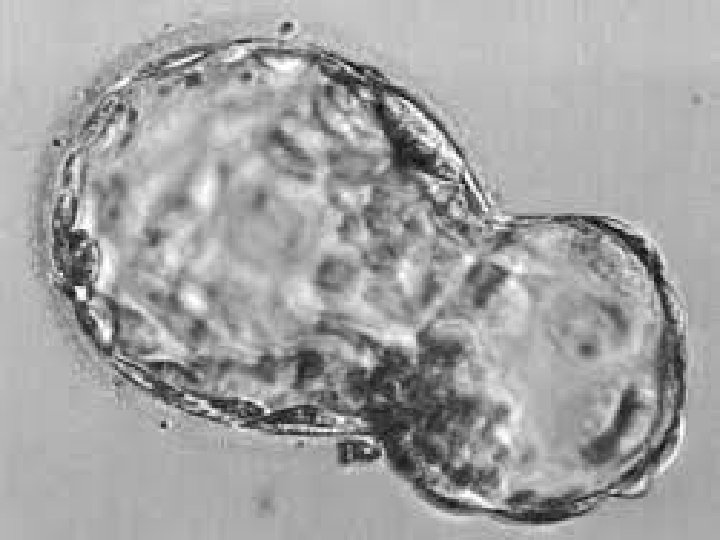
Blastomere Biopsy






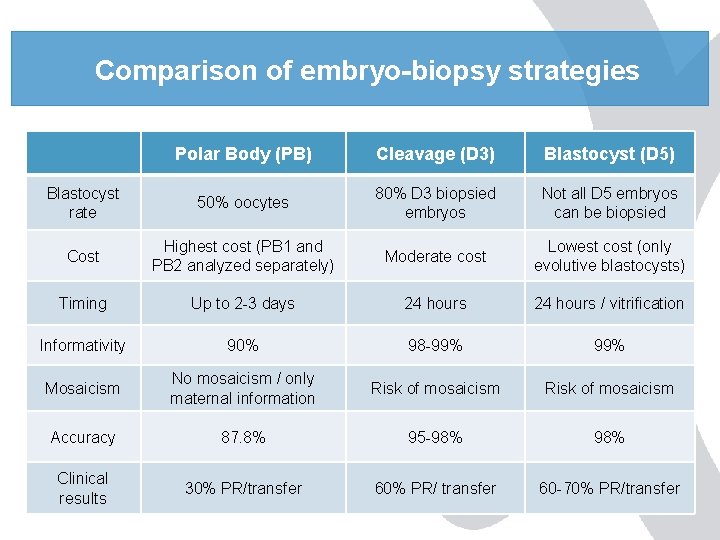
Comparison of embryo-biopsy strategies Polar Body (PB) Cleavage (D 3) Blastocyst (D 5) Blastocyst rate 50% oocytes 80% D 3 biopsied embryos Not all D 5 embryos can be biopsied Cost Highest cost (PB 1 and PB 2 analyzed separately) Moderate cost Lowest cost (only evolutive blastocysts) Timing Up to 2 -3 days 24 hours / vitrification Informativity 90% 98 -99% Mosaicism No mosaicism / only maternal information Risk of mosaicism Accuracy 87. 8% 95 -98% Clinical results 30% PR/transfer 60% PR/ transfer 60 -70% PR/transfer

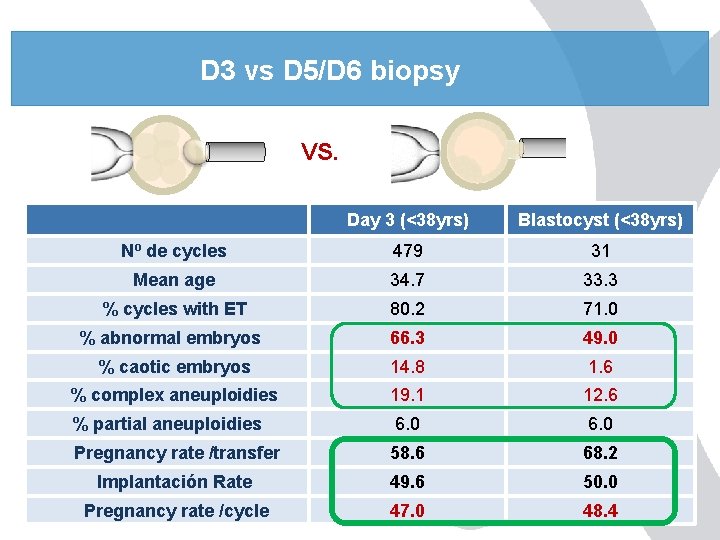
D 3 vs D 5/D 6 biopsy VS. Day 3 (<38 yrs) Blastocyst (<38 yrs) Nº de cycles 479 31 Mean age 34. 7 33. 3 % cycles with ET 80. 2 71. 0 % abnormal embryos 66. 3 49. 0 % caotic embryos 14. 8 1. 6 % complex aneuploidies 19. 1 12. 6 % partial aneuploidies 6. 0 Pregnancy rate /transfer 58. 6 68. 2 Implantación Rate 49. 6 50. 0 Pregnancy rate /cycle 47. 0 48. 4

Aim of PGD • Offers couples at risk the chance to have an unaffected (desired) child , without facing termination of pregnancy.

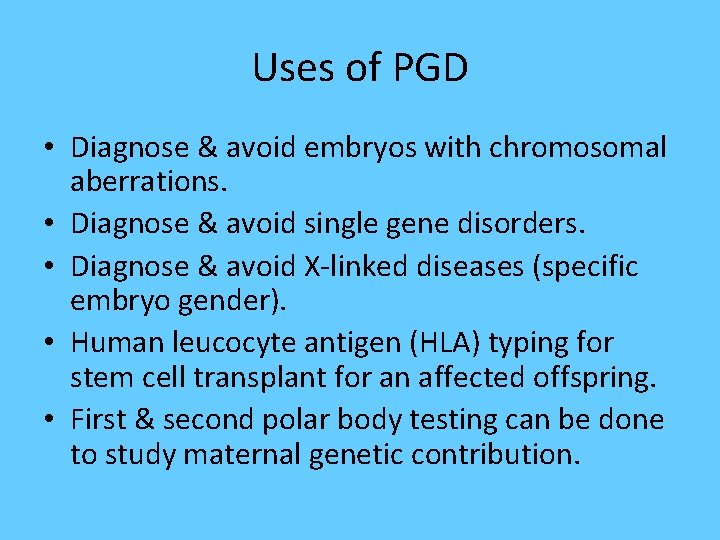
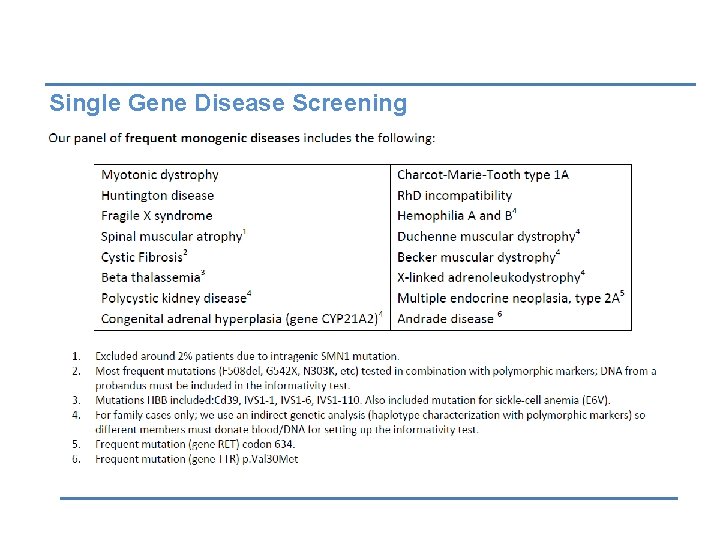
Uses of PGD • Diagnose & avoid embryos with chromosomal aberrations. • Diagnose & avoid single gene disorders. • Diagnose & avoid X-linked diseases (specific embryo gender). • Human leucocyte antigen (HLA) typing for stem cell transplant for an affected offspring. • First & second polar body testing can be done to study maternal genetic contribution.

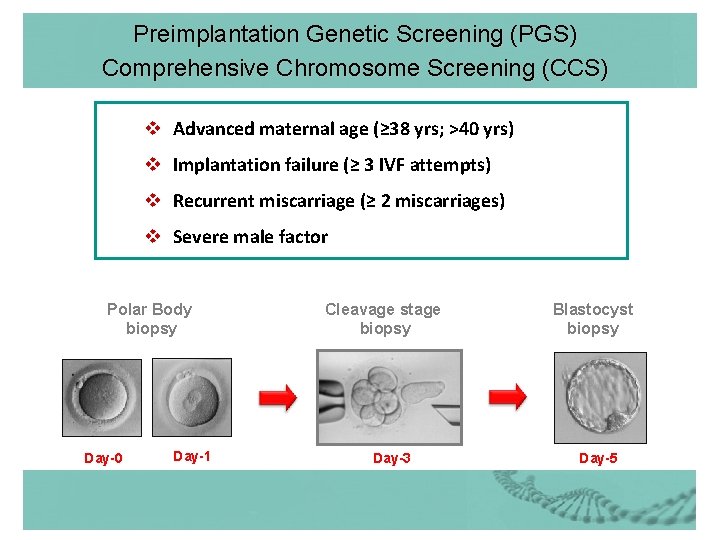
Preimplantation Genetic Screening (PGS) Comprehensive Chromosome Screening (CCS) v Advanced maternal age (≥ 38 yrs; >40 yrs) v Implantation failure (≥ 3 IVF attempts) v Recurrent miscarriage (≥ 2 miscarriages) v Severe male factor Polar Body biopsy Day-0 Day-1 Cleavage stage biopsy Day-3 Blastocyst biopsy Day-5

Professional Pre Requisites • Parents counseled & accepted. • ≥ 5 grade A or 1 embryos. • Both IVF & genetic labs are trained & prepared to do PGD.

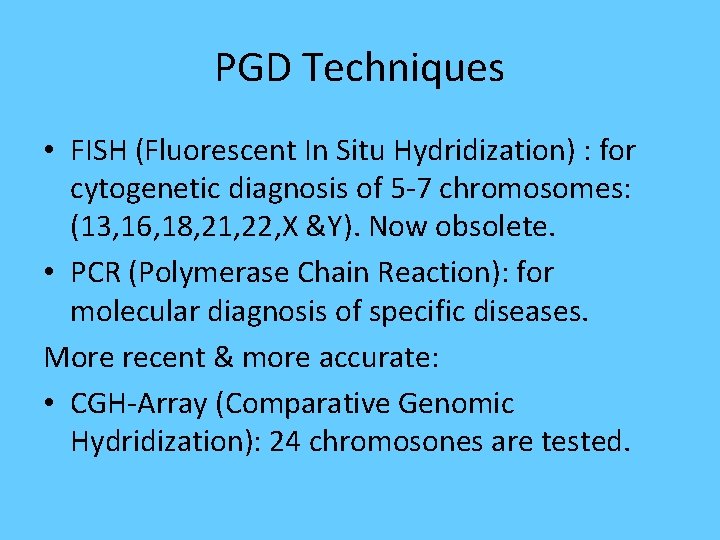
PGD Techniques • FISH (Fluorescent In Situ Hydridization) : for cytogenetic diagnosis of 5 -7 chromosomes: (13, 16, 18, 21, 22, X &Y). Now obsolete. • PCR (Polymerase Chain Reaction): for molecular diagnosis of specific diseases. More recent & more accurate: • CGH-Array (Comparative Genomic Hydridization): 24 chromosones are tested.

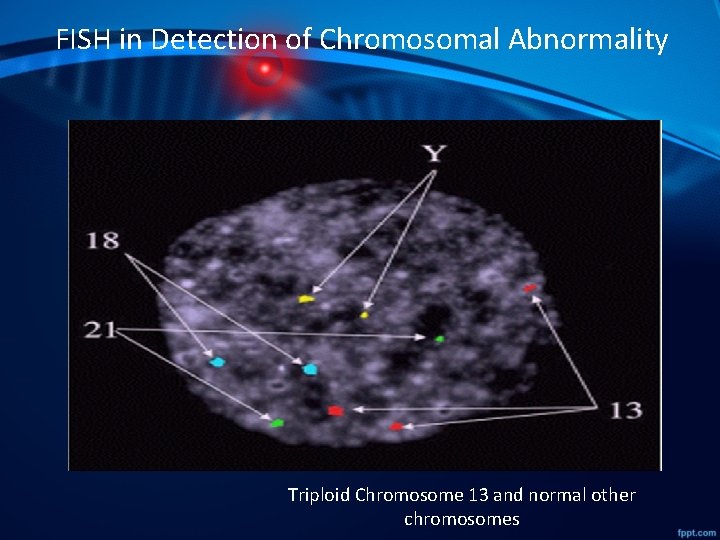
FISH in Detection of Chromosomal Abnormality Triploid Chromosome 13 and normal other chromosomes

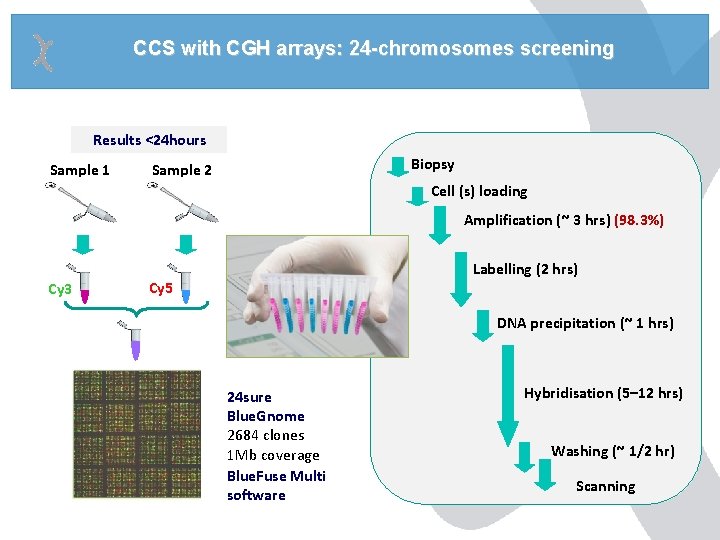
CCS with CGH arrays: 24 -chromosomes screening Results <24 hours Sample 1 Biopsy Sample 2 Cell (s) loading Amplification (~ 3 hrs) (98. 3%) Cy 3 Labelling (2 hrs) Cy 5 DNA precipitation (~ 1 hrs) 24 sure Blue. Gnome 2684 clones 1 Mb coverage Blue. Fuse Multi software Hybridisation (5– 12 hrs) Washing (~ 1/2 hr) Scanning

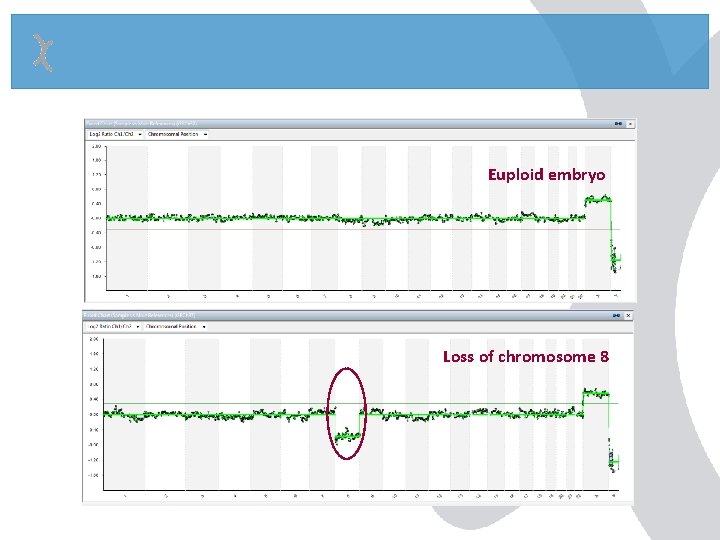
Euploid embryo Loss of chromosome 8

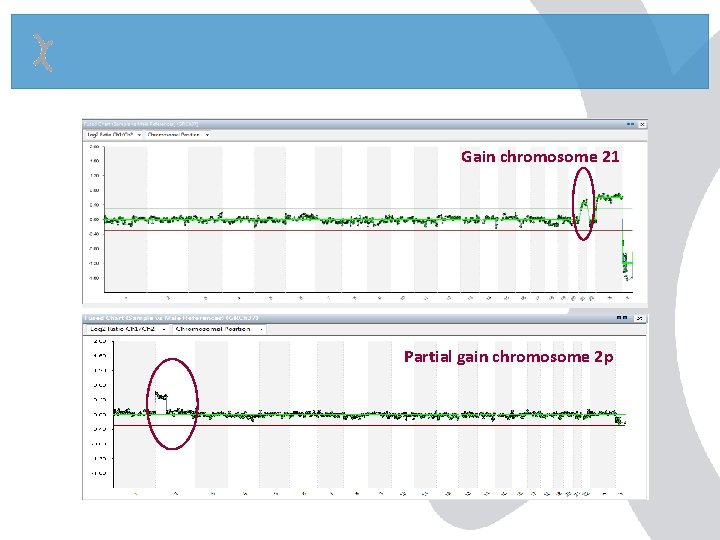
Gain chromosome 21 Partial gain chromosome 2 p

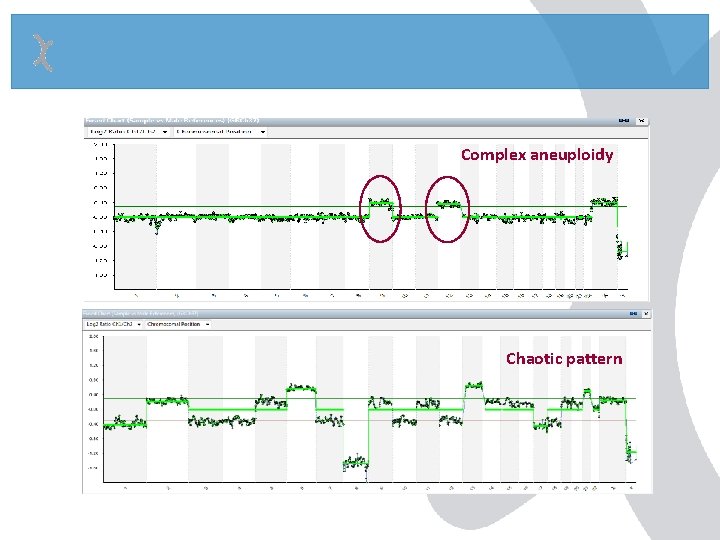
Complex aneuploidy Chaotic pattern

Ethical & Legal Issues • Couple’s Informed consent. • Should not be done on social grounds. • Should be done on professional grounds only. • Should be provided only by well trained teams

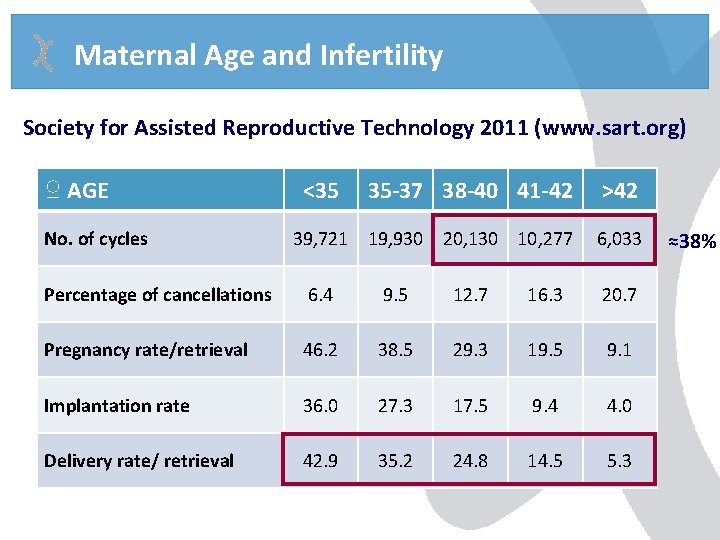
Maternal Age and Infertility Society for Assisted Reproductive Technology 2011 (www. sart. org) ♀ AGE No. of cycles <35 35 -37 38 -40 41 -42 >42 39, 721 19, 930 20, 130 10, 277 6, 033 Percentage of cancellations 6. 4 9. 5 12. 7 16. 3 20. 7 Pregnancy rate/retrieval 46. 2 38. 5 29. 3 19. 5 9. 1 Implantation rate 36. 0 27. 3 17. 5 9. 4 4. 0 Delivery rate/ retrieval 42. 9 35. 2 24. 8 14. 5 5. 3 ≈38%

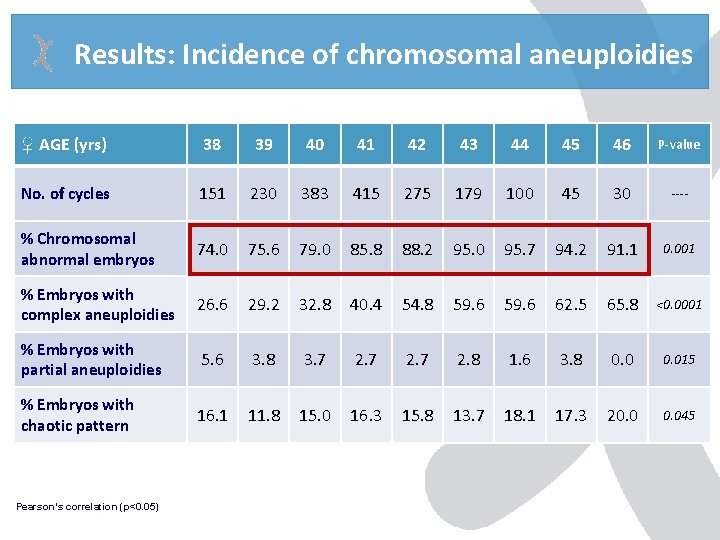
Results: Incidence of chromosomal aneuploidies ♀ AGE (yrs) 38 39 40 41 42 43 44 45 46 P-value No. of cycles 151 230 383 415 275 179 100 45 30 ---- % Chromosomal abnormal embryos 74. 0 75. 6 79. 0 85. 8 88. 2 95. 0 95. 7 94. 2 91. 1 0. 001 % Embryos with complex aneuploidies 26. 6 29. 2 32. 8 40. 4 54. 8 59. 6 62. 5 65. 8 <0. 0001 % Embryos with partial aneuploidies 5. 6 3. 8 3. 7 2. 8 1. 6 3. 8 0. 015 % Embryos with chaotic pattern 16. 1 11. 8 15. 0 16. 3 15. 8 13. 7 18. 1 17. 3 20. 045 Pearson’s correlation (p<0. 05)

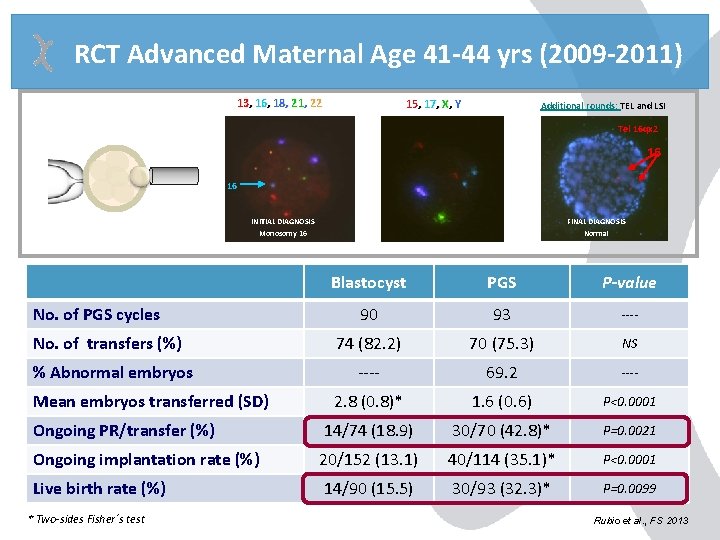
RCT Advanced Maternal Age 41 -44 yrs (2009 -2011) 13, 16, 18, 21, 22 15, 17, X, Y Additional rounds: TEL and LSI Tel 16 qx 2 16 16 INITIAL DIAGNOSIS Monosomy 16 FINAL DIAGNOSIS Normal Blastocyst PGS P-value 90 93 ---- 74 (82. 2) 70 (75. 3) NS ---- 69. 2 ---- 2. 8 (0. 8)* 1. 6 (0. 6) P<0. 0001 Ongoing PR/transfer (%) 14/74 (18. 9) 30/70 (42. 8)* P=0. 0021 Ongoing implantation rate (%) 20/152 (13. 1) 40/114 (35. 1)* P<0. 0001 Live birth rate (%) 14/90 (15. 5) 30/93 (32. 3)* P=0. 0099 No. of PGS cycles No. of transfers (%) % Abnormal embryos Mean embryos transferred (SD) * Two-sides Fisher´s test Rubio et al. , FS 2013

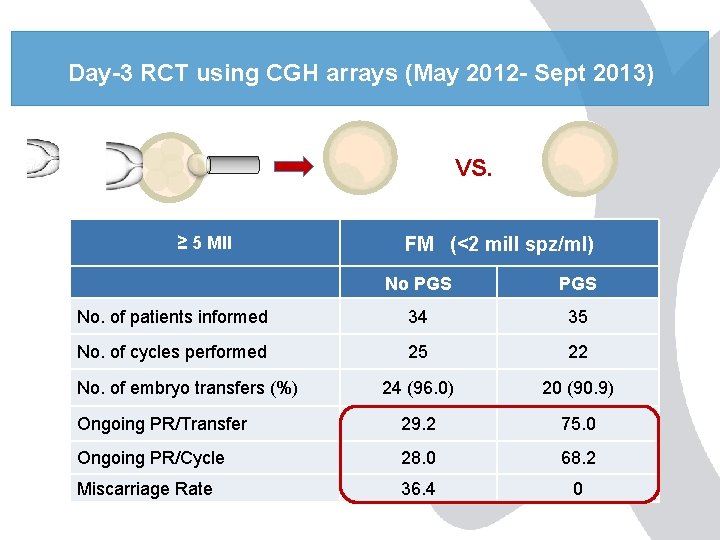
Day-3 RCT using CGH arrays (May 2012 - Sept 2013) VS. ≥ 5 MII FM (<2 mill spz/ml) No PGS No. of patients informed 34 35 No. of cycles performed 25 22 24 (96. 0) 20 (90. 9) Ongoing PR/Transfer 29. 2 75. 0 Ongoing PR/Cycle 28. 0 68. 2 Miscarriage Rate 36. 4 0 No. of embryo transfers (%)

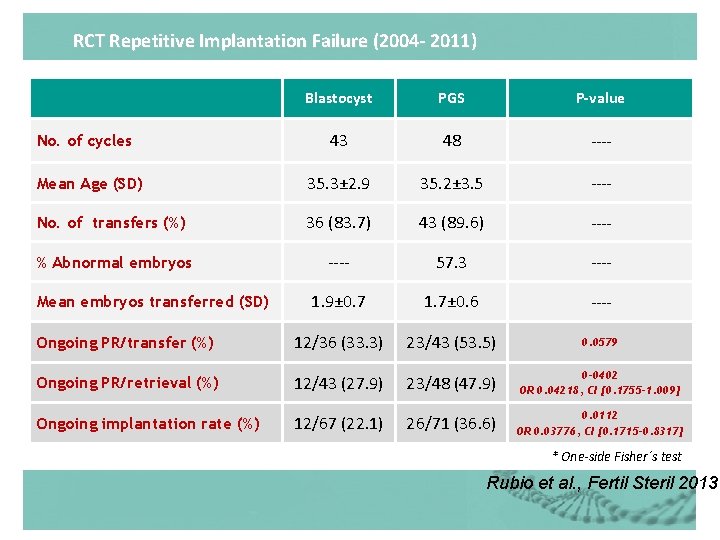
RCT Repetitive Implantation Failure (2004 - 2011) Blastocyst PGS P-value 43 48 ---- Mean Age (SD) 35. 3± 2. 9 35. 2± 3. 5 ---- No. of transfers (%) 36 (83. 7) 43 (89. 6) ---- % Abnormal embryos ---- 57. 3 ---- 1. 9± 0. 7 1. 7± 0. 6 ---- Ongoing PR/transfer (%) 12/36 (33. 3) 23/43 (53. 5) 0. 0579 Ongoing PR/retrieval (%) 12/43 (27. 9) 23/48 (47. 9) 0 -0402 OR 0. 04218, CI [0. 1755 -1. 009] Ongoing implantation rate (%) 12/67 (22. 1) 26/71 (36. 6) 0. 0112 OR 0. 03776, CI [0. 1715 -0. 8317] No. of cycles Mean embryos transferred (SD) * One-side Fisher´s test Rubio et al. , Fertil Steril 2013

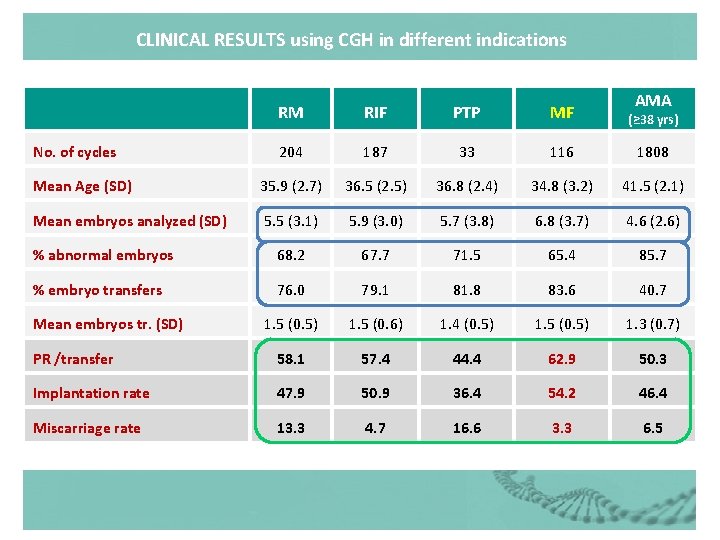
CLINICAL RESULTS using CGH in different indications AMA RM RIF PTP MF (≥ 38 yrs) 204 187 33 116 1808 Mean Age (SD) 35. 9 (2. 7) 36. 5 (2. 5) 36. 8 (2. 4) 34. 8 (3. 2) 41. 5 (2. 1) Mean embryos analyzed (SD) 5. 5 (3. 1) 5. 9 (3. 0) 5. 7 (3. 8) 6. 8 (3. 7) 4. 6 (2. 6) % abnormal embryos 68. 2 67. 7 71. 5 65. 4 85. 7 % embryo transfers 76. 0 79. 1 81. 8 83. 6 40. 7 1. 5 (0. 5) 1. 5 (0. 6) 1. 4 (0. 5) 1. 5 (0. 5) 1. 3 (0. 7) PR /transfer 58. 1 57. 4 44. 4 62. 9 50. 3 Implantation rate 47. 9 50. 9 36. 4 54. 2 46. 4 Miscarriage rate 13. 3 4. 7 16. 6 3. 3 6. 5 No. of cycles Mean embryos tr. (SD)

Single Gene Disease Screening (Monogenic Diseases)

PCR DNA CHROMOSOME DOUBLE HELIX

Single Gene Disease Screening

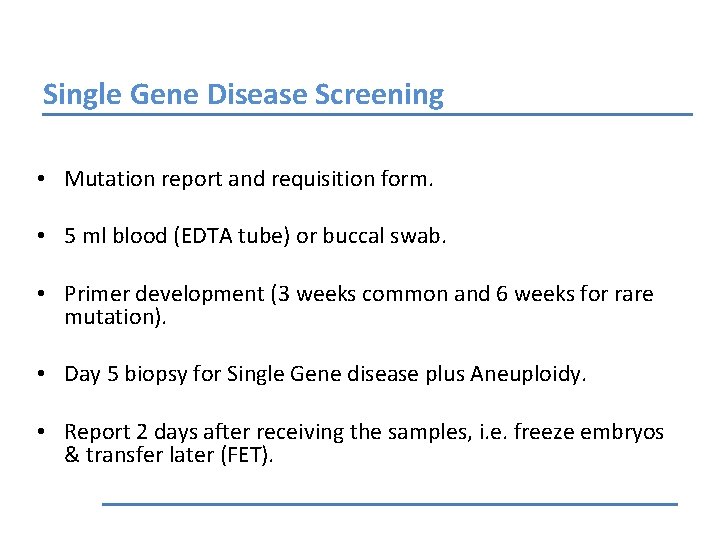
Single Gene Disease Screening • Mutation report and requisition form. • 5 ml blood (EDTA tube) or buccal swab. • Primer development (3 weeks common and 6 weeks for rare mutation). • Day 5 biopsy for Single Gene disease plus Aneuploidy. • Report 2 days after receiving the samples, i. e. freeze embryos & transfer later (FET).

Children Follow up • PGD Children follow up showed similar outcome to IVF / ICSI without PGD.

Near Future of PDG Next Generation Sequencing (NGS) Rapid Accurate More efficient Scan for more genes

Work in progress: NGS Illumina Sept 2013

Work in progress: NGS Life Tech November 2013

Conclusions • PGD is currently easier & faster to do, cheaper, more comprehensive & accurate than before. • It is now an integral part of ART. • More advances in the technology is coming soon. • It should be use within clear regulations. • It has a great potential in reducing incidence of chromosomal & genetic disorders.

Future of Genetics • Hopefully in the near future we will wetness the use of knowledge & technologies in genetics to repair or correct genetic defects.

Thanks Finally I would like to thank Prof. Carlos Simon Scientific Director of IVI & Igenomix Valencia , Spain For allowing me to share some of his results in this lecture.

Thank you