PREDICTING THE PRODUCTS OF SINGLE DISPLACEMENT REACTIONS Single






- Slides: 9

PREDICTING THE PRODUCTS OF SINGLE DISPLACEMENT REACTIONS

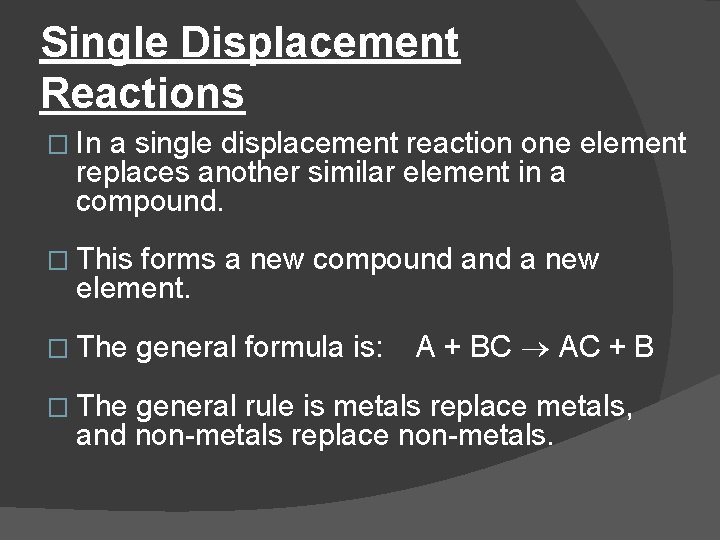
Single Displacement Reactions � In a single displacement reaction one element replaces another similar element in a compound. � This forms a new compound a new element. � The general formula is: A + BC AC + B � The general rule is metals replace metals, and non-metals replace non-metals.

The Activity Series � The activity series is a list of elements and their order of decreasing reactivity. � On the metal series, a metal element will displace a metal ion that appears below it in the series. � For a reaction to occur, the lone metal must be higher on the activity series.

Halogens Activity Series � On the halogen series, a halogen will displace another halogen that is lower on the series. � Fluorine is the most reactive. � Iodine is the least reactive.

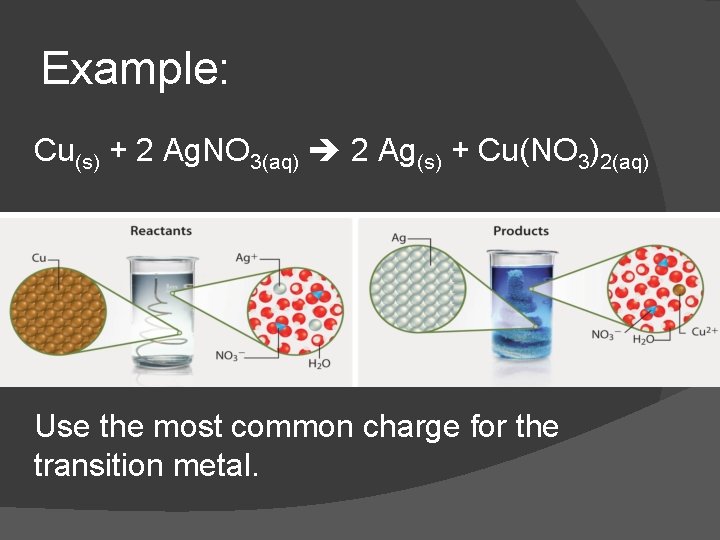
Example: Cu(s) + 2 Ag. NO 3(aq) 2 Ag(s) + Cu(NO 3)2(aq) Use the most common charge for the transition metal.

Practice �Na(s) + Zn. Cl 2(aq) �Na is higher on the Activity Series than Zn, therefore a reaction will occur. 2 Na(s) + Zn. Cl 2(aq) Zn(s) + 2 Na. Cl(aq)

� Li(s) + HNO 3(aq) �Li is higher on the Activity Series than H, therefore a reaction will occur. 2 Li(s) + 2 HNO 3(aq) H 2(g) + 2 Li. NO 3(aq)

�Br 2(l) + KI(aq) �Br is higher on the Activity Series than I, therefore a reaction will occur. Br 2(l) + 2 KI(aq) I 2(s) + 2 KBr(aq)

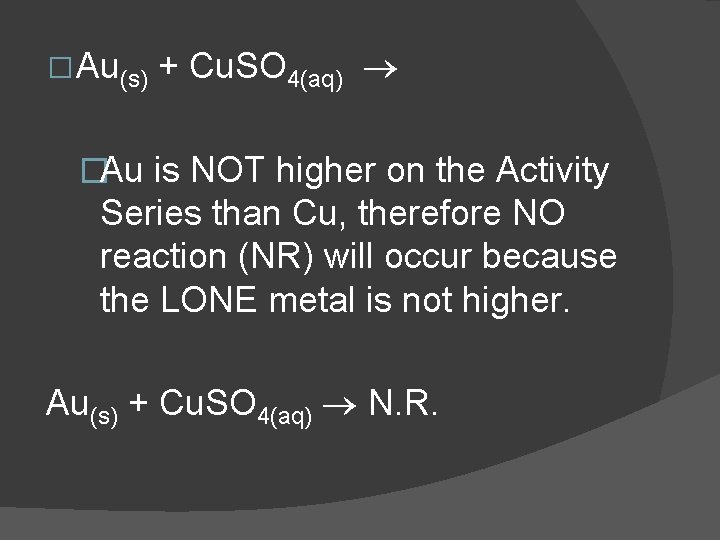
� Au(s) + Cu. SO 4(aq) �Au is NOT higher on the Activity Series than Cu, therefore NO reaction (NR) will occur because the LONE metal is not higher. Au(s) + Cu. SO 4(aq) N. R.
 Predicting products of chemical reactions
Predicting products of chemical reactions Synthesis reaction predicting products
Synthesis reaction predicting products Combination reaction equation
Combination reaction equation Predicting products of chemical reactions
Predicting products of chemical reactions Single replacement activity series
Single replacement activity series Single replacement products
Single replacement products Single displacement vs double displacement
Single displacement vs double displacement Stoichiometry predicting amounts in reactions
Stoichiometry predicting amounts in reactions Predicting redox reactions
Predicting redox reactions Predicting precipitation reactions
Predicting precipitation reactions