Predicting the Products of Chemical Reactions Refresher Chemistry
- Slides: 9

Predicting the Products of Chemical Reactions Refresher Chemistry

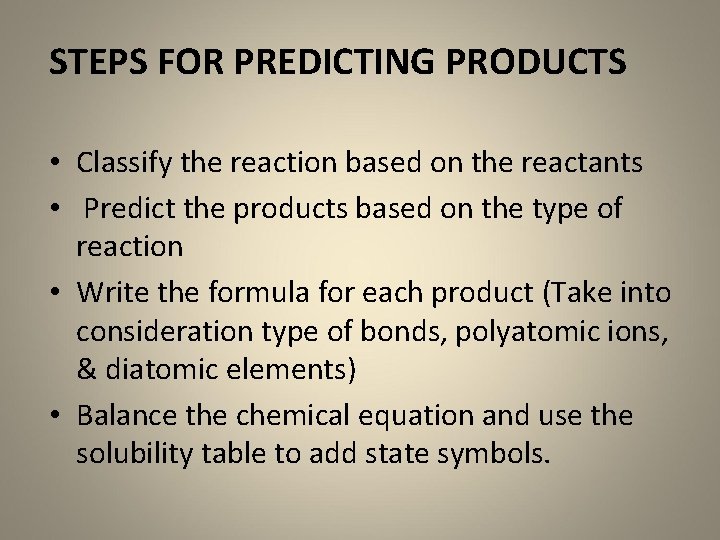
STEPS FOR PREDICTING PRODUCTS • Classify the reaction based on the reactants • Predict the products based on the type of reaction • Write the formula for each product (Take into consideration type of bonds, polyatomic ions, & diatomic elements) • Balance the chemical equation and use the solubility table to add state symbols.

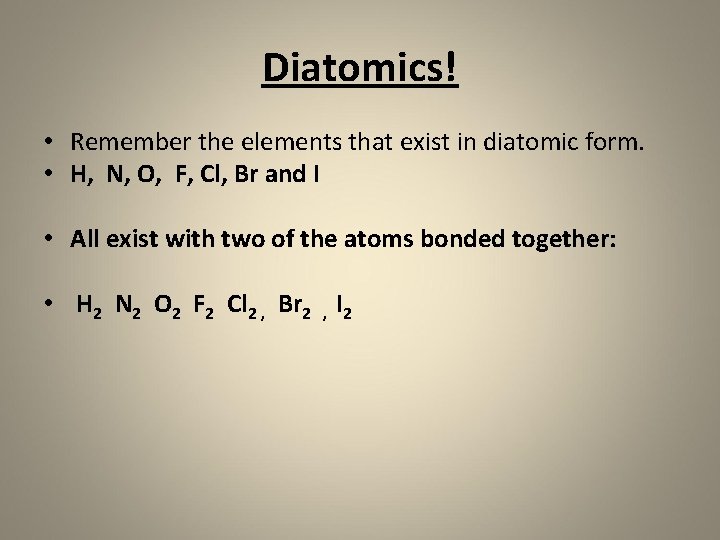
Diatomics! • Remember the elements that exist in diatomic form. • H, N, O, F, Cl, Br and I • All exist with two of the atoms bonded together: • H 2 N 2 O 2 F 2 Cl 2 , Br 2 , I 2

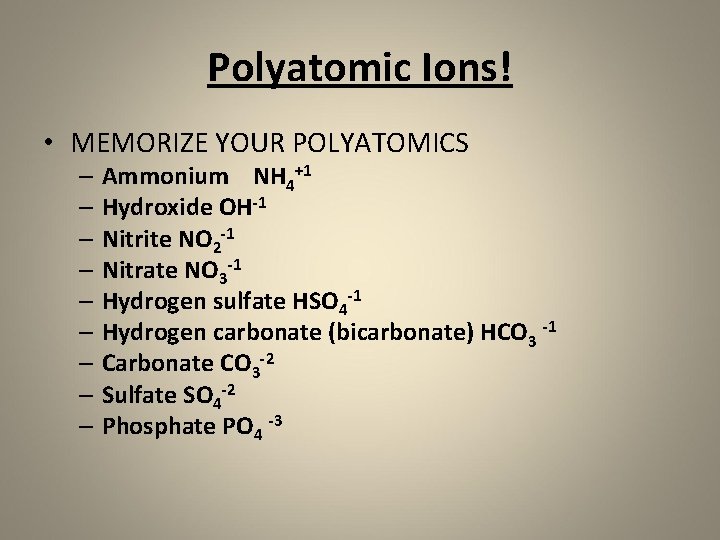
Polyatomic Ions! • MEMORIZE YOUR POLYATOMICS – Ammonium NH 4+1 – Hydroxide OH-1 – Nitrite NO 2 -1 – Nitrate NO 3 -1 – Hydrogen sulfate HSO 4 -1 – Hydrogen carbonate (bicarbonate) HCO 3 -1 – Carbonate CO 3 -2 – Sulfate SO 4 -2 – Phosphate PO 4 -3

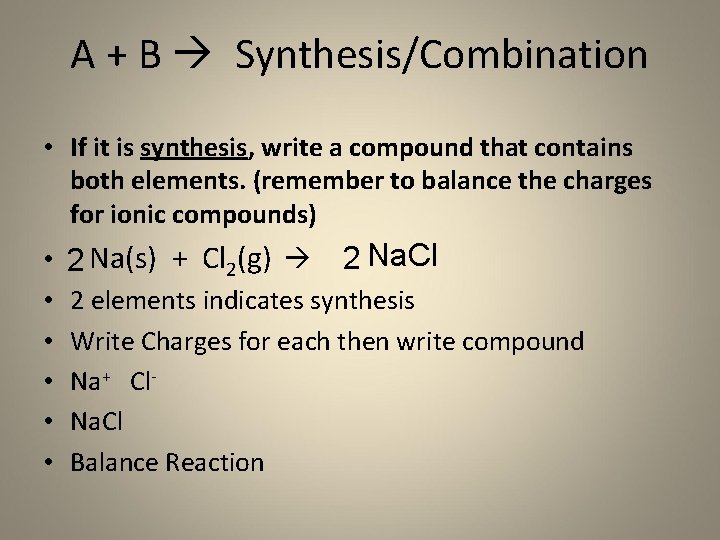
A + B Synthesis/Combination • If it is synthesis, write a compound that contains both elements. (remember to balance the charges for ionic compounds) • • • 2 Na(s) + Cl 2(g) 2 Na. Cl 2 elements indicates synthesis Write Charges for each then write compound Na+ Cl. Na. Cl Balance Reaction

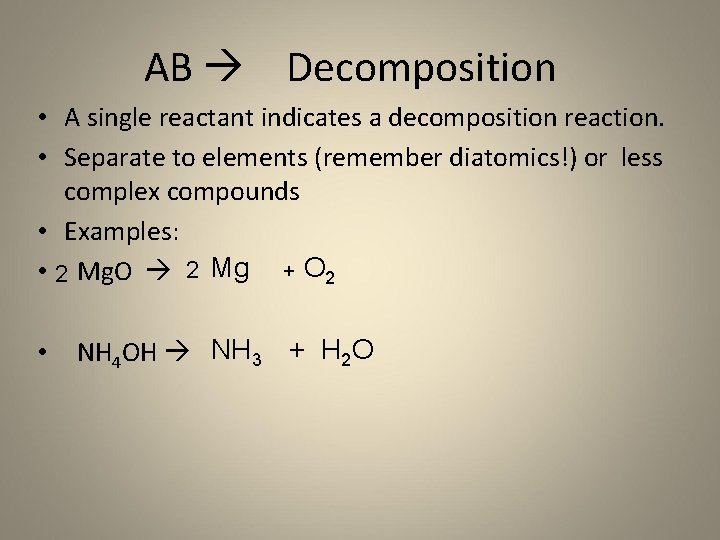
AB Decomposition • A single reactant indicates a decomposition reaction. • Separate to elements (remember diatomics!) or less complex compounds • Examples: • 2 Mg. O 2 Mg + O 2 • NH 4 OH NH 3 + H 2 O

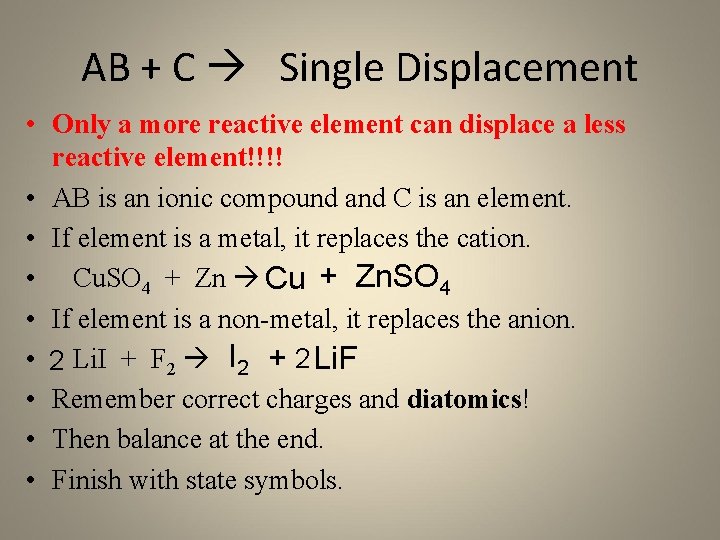
AB + C Single Displacement • Only a more reactive element can displace a less reactive element!!!! • AB is an ionic compound and C is an element. • If element is a metal, it replaces the cation. • Cu. SO 4 + Zn Cu + Zn. SO 4 • If element is a non-metal, it replaces the anion. • 2 Li. I + F 2 I 2 + 2 Li. F • Remember correct charges and diatomics! • Then balance at the end. • Finish with state symbols.

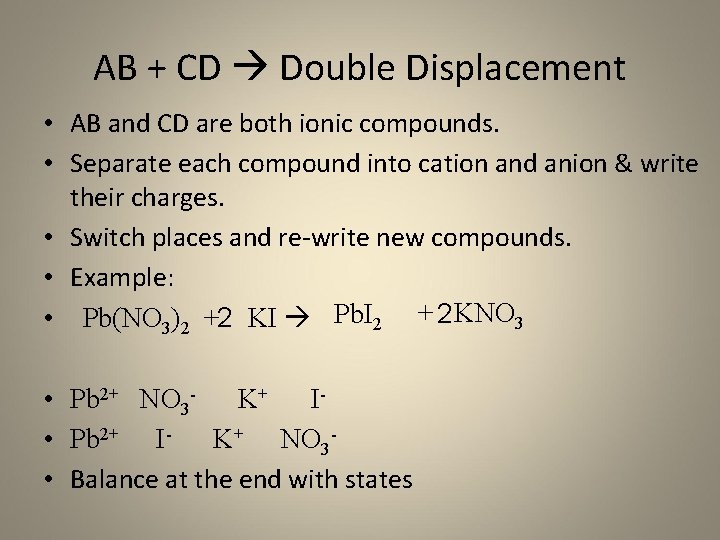
AB + CD Double Displacement • AB and CD are both ionic compounds. • Separate each compound into cation and anion & write their charges. • Switch places and re-write new compounds. • Example: • Pb(NO 3)2 +2 KI Pb. I 2 + 2 KNO 3 • Pb 2+ NO 3 - K+ I • Pb 2+ I- K+ NO 3 • Balance at the end with states

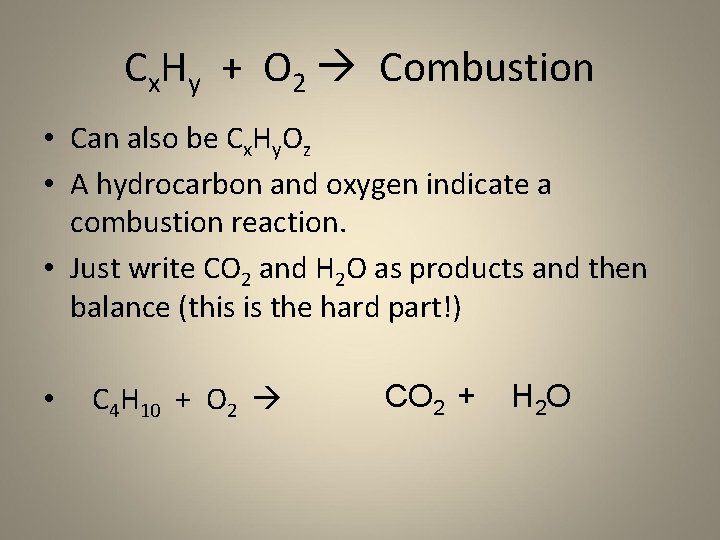
Cx. Hy + O 2 Combustion • Can also be Cx. Hy. Oz • A hydrocarbon and oxygen indicate a combustion reaction. • Just write CO 2 and H 2 O as products and then balance (this is the hard part!) • C 4 H 10 + O 2 CO 2 + H 2 O