Predicting Reactions General Rules 1 All reactions occur






- Slides: 10

Predicting Reactions General Rules 1. All reactions occur 2. Net ionic reactions only 3. Phases are not required : aqueous by charge 4. Completely dissociated chemicals shown as ions (i. e. strong acid is H+ & A-) 5. All other dissociated chemicals shown as complete compound (i. e. weak acid is HA) 1

Predicting Reactions General Rules (Pg 2) 6. “Burned in air” - means combustion in O 2 7. “Heated” means decomposition 2

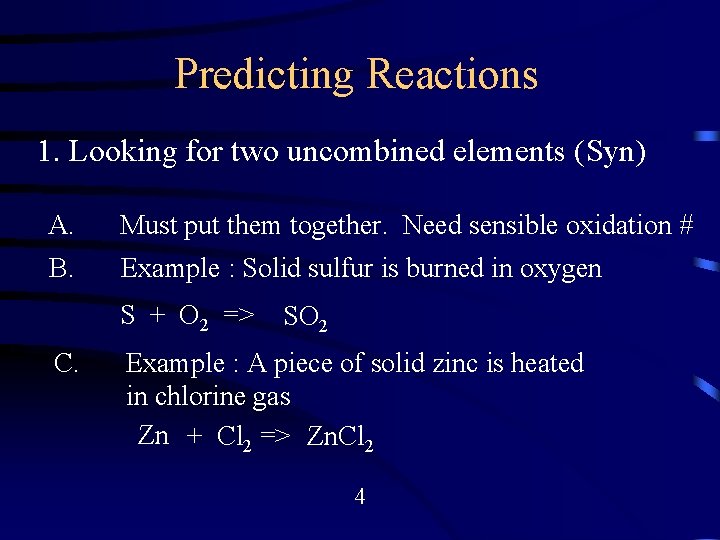
Predicting Reactions Major Types 1. 2. 3. 4. 5. 6. 7. 8. Look for two uncombined elements (Syn. ) Look for single reactant (Decomposition) Look for Combustion Reaction Look for Acid-Base Reactions (DR) Look for Two Salt Solution (Precip) Look for REDOX Reaction Look for water as Reactant Look for Transitional metal and Ligands 3

Predicting Reactions 1. Looking for two uncombined elements (Syn) A. Must put them together. Need sensible oxidation # B. Example : Solid sulfur is burned in oxygen S + O 2 => C. SO 2 Example : A piece of solid zinc is heated in chlorine gas Zn + Cl 2 => Zn. Cl 2 4

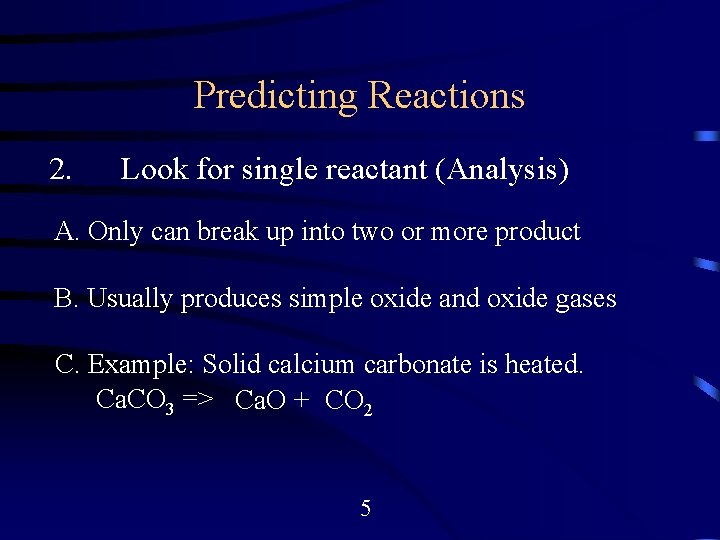
Predicting Reactions 2. Look for single reactant (Analysis) A. Only can break up into two or more product B. Usually produces simple oxide and oxide gases C. Example: Solid calcium carbonate is heated. Ca. CO 3 => Ca. O + CO 2 5

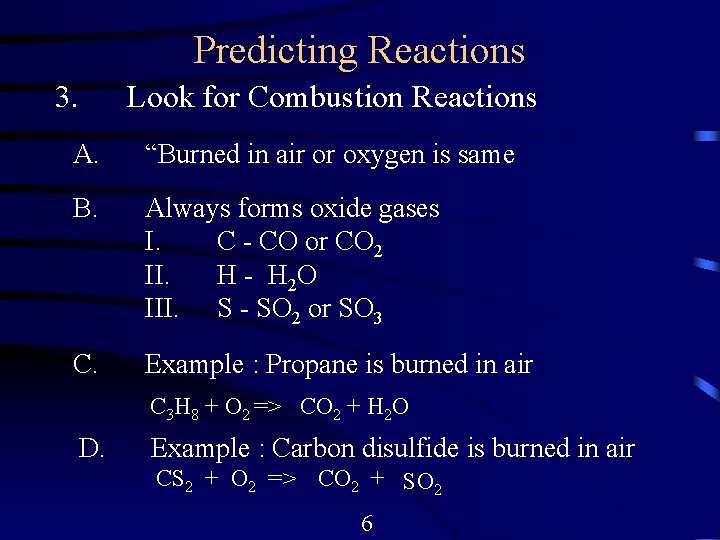
Predicting Reactions 3. Look for Combustion Reactions A. “Burned in air or oxygen is same B. Always forms oxide gases I. C - CO or CO 2 II. H - H 2 O III. S - SO 2 or SO 3 C. Example : Propane is burned in air C 3 H 8 + O 2 => CO 2 + H 2 O D. Example : Carbon disulfide is burned in air CS 2 + O 2 => CO 2 + SO 2 6

Predicting Reaction 4. Looking for Acid-Base Reaction A. Acid and Base Be careful : only strong acid & strong base give H+ + OH- => H 2 O Weak acid/base : can’t get rid of other part Example: Solution of hydrofluoric acid & sodium hydroxide are mixed HF + OH- => F- + H 2 O (must keep F- ) 7

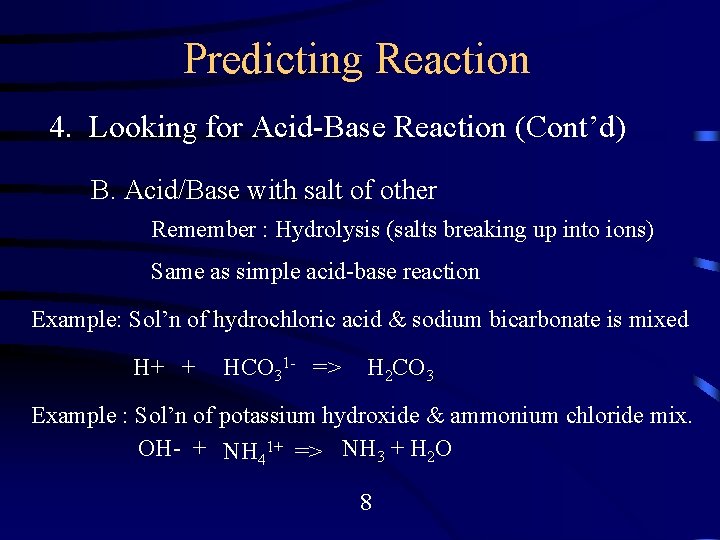
Predicting Reaction 4. Looking for Acid-Base Reaction (Cont’d) B. Acid/Base with salt of other Remember : Hydrolysis (salts breaking up into ions) Same as simple acid-base reaction Example: Sol’n of hydrochloric acid & sodium bicarbonate is mixed H+ + HCO 31 - => H 2 CO 3 Example : Sol’n of potassium hydroxide & ammonium chloride mix. OH- + NH 41+ => NH 3 + H 2 O 8

Predicting Reaction 4. Looking for Acid-Base Reaction (Cont’d) C. Polyprotic acid & Sulfuric acid Polyprotic : Only one H+ comes off or on Exception: “Excess acid”- goes all the way Sulfuric acid - For now, assume both concentrated & dilute form completely dissociate Example: “Equal volume” of “equimolar” solutions of phosphoric acid & potassium hydroxide are mixed H 3 PO 4 + OH 1 - => H 2 PO 41 - + H 2 O Example : Excess hydrochloric acid mixed w/sol’n potassium sulfide H+ + S 2 - => H 2 S 9

Predicting Reaction 5. Looking for Two Salt Solution A. General Assumptions (Does not replace solubility rules) I. A salt containing (1 - charge) anion is SOLUBLE (don’t forget solubility rules especially Ag. Cl) II. A salt containing (2 - or 3 - charge) anion : INSOLUBLE (don’t forget solubility rules especially with sulfate) Example: A sol’n of silver nitrate is added to potassium iodide sol’n Ag+ + I 1 - => Ag. I (Remember reaction must occur) 10