Predicting Products Solubility Rules Double Replacement Double 4

Predicting Products Solubility Rules
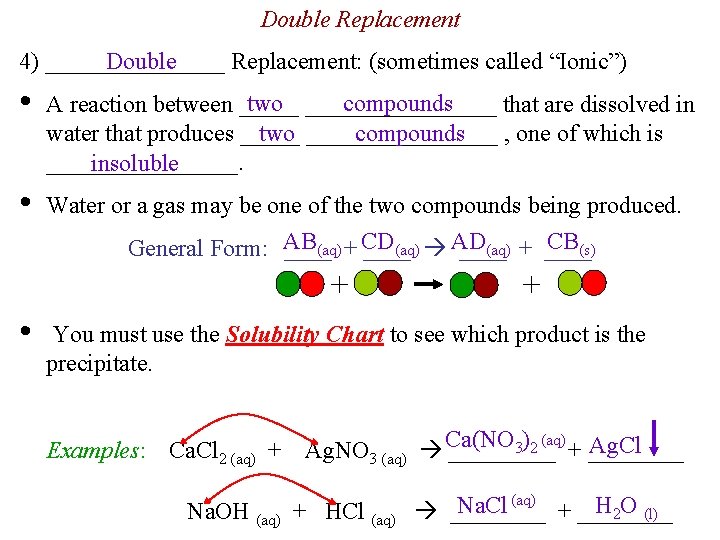
Double Replacement Double 4) ________ Replacement: (sometimes called “Ionic”) • two ________ compounds A reaction between _____ that are dissolved in two ________ compounds water that produces _____ , one of which is ________. insoluble • Water or a gas may be one of the two compounds being produced. CB(s) (aq) + CD (aq) AD (aq) + ____ General Form: AB ____ + • + You must use the Solubility Chart to see which product is the precipitate. Examples: Ca. Cl 2 (aq) + 3)2 (aq) + ____ Ag. Cl Ag. NO 3 (aq) Ca(NO _____ Na. Cl (aq) + ____ H 2 O (l) Na. OH (aq) + HCl (aq) ____
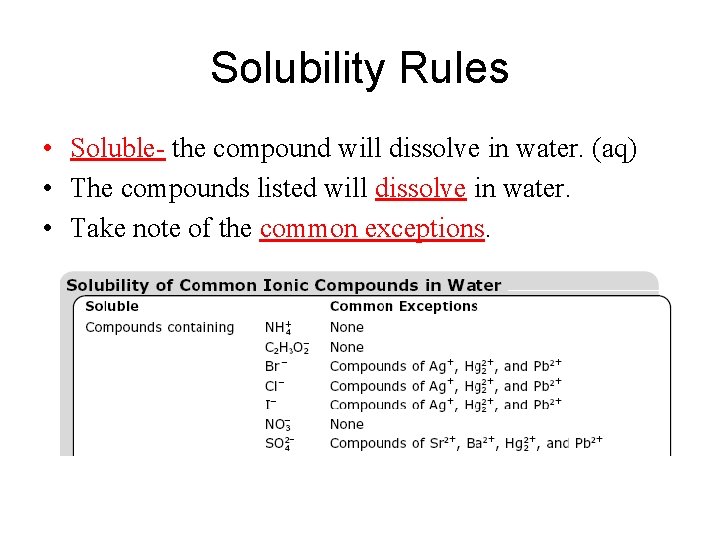
Solubility Rules • Soluble- the compound will dissolve in water. (aq) • The compounds listed will dissolve in water. • Take note of the common exceptions.
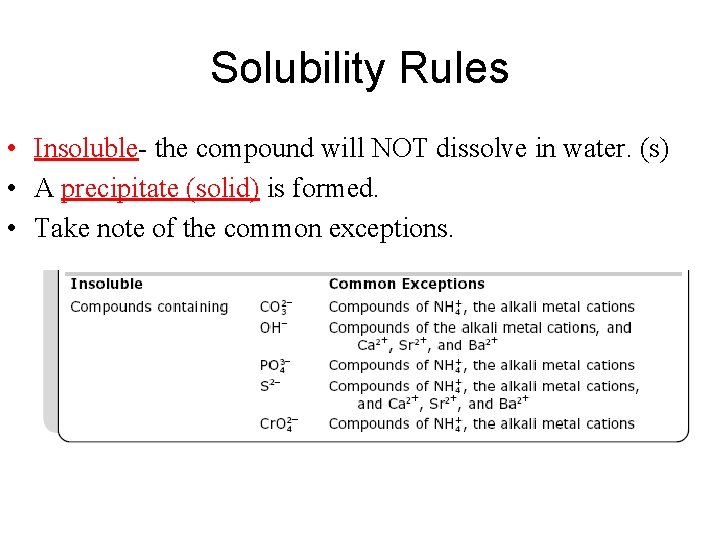
Solubility Rules • Insoluble- the compound will NOT dissolve in water. (s) • A precipitate (solid) is formed. • Take note of the common exceptions.
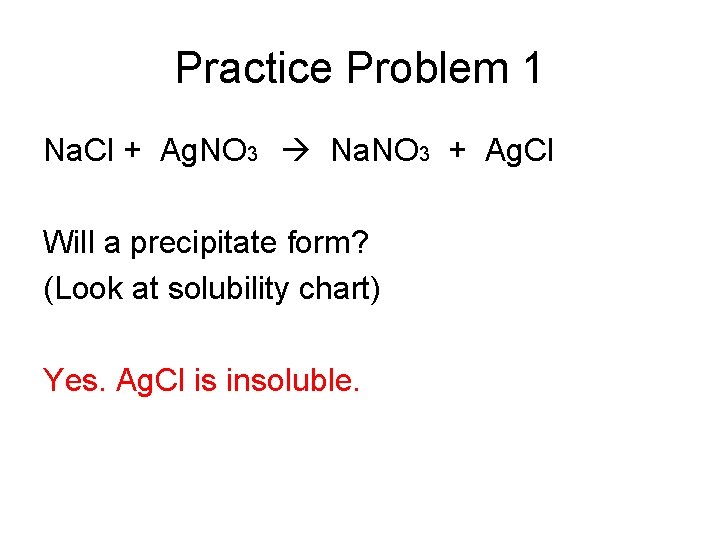
Practice Problem 1 Na. Cl + Ag. NO 3 Na. NO 3 + Ag. Cl Will a precipitate form? (Look at solubility chart) Yes. Ag. Cl is insoluble.
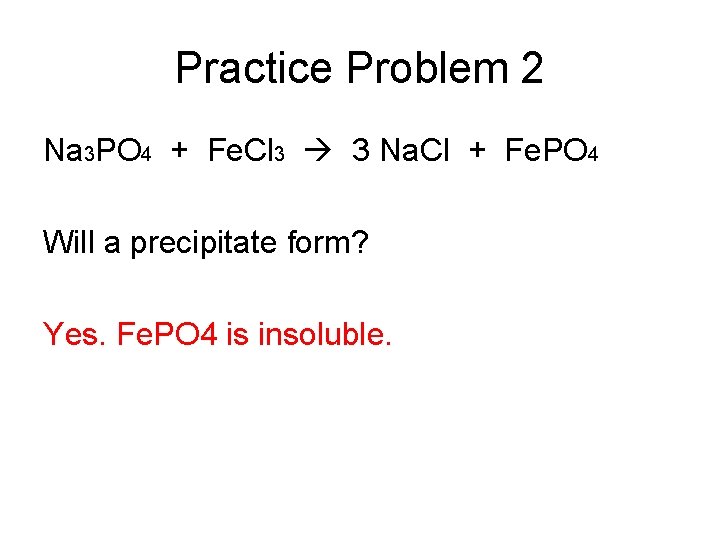
Practice Problem 2 Na 3 PO 4 + Fe. Cl 3 3 Na. Cl + Fe. PO 4 Will a precipitate form? Yes. Fe. PO 4 is insoluble.
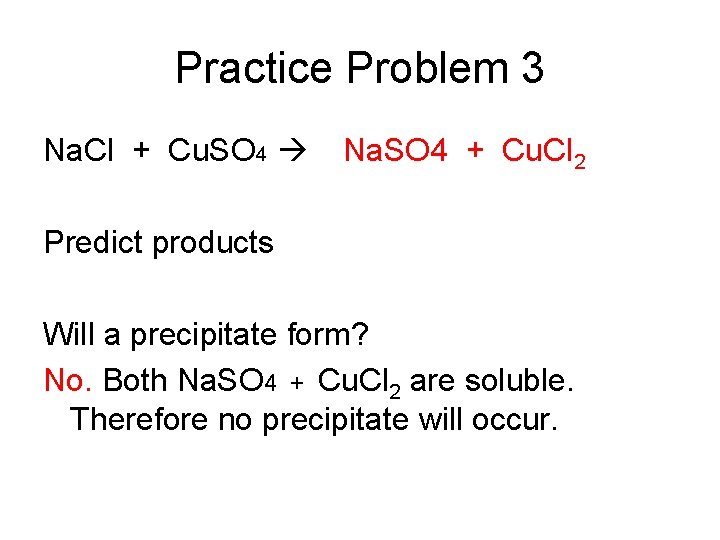
Practice Problem 3 Na. Cl + Cu. SO 4 Na. SO 4 + Cu. Cl 2 Predict products Will a precipitate form? No. Both Na. SO 4 + Cu. Cl 2 are soluble. Therefore no precipitate will occur.
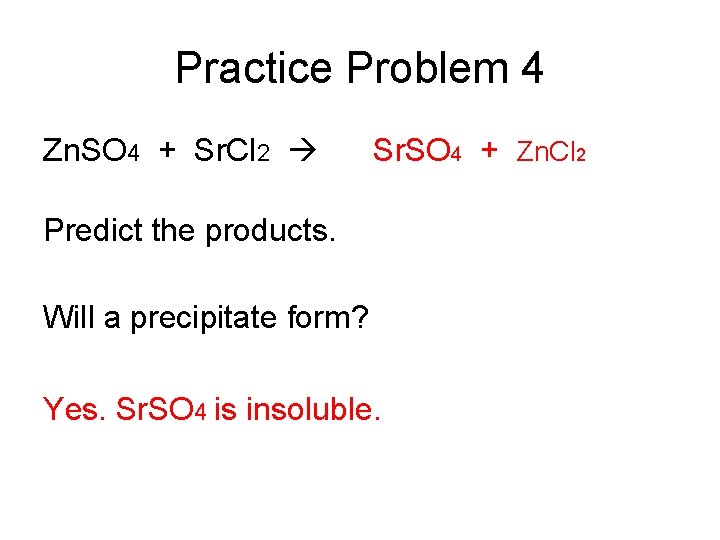
Practice Problem 4 Zn. SO 4 + Sr. Cl 2 Sr. SO 4 + Zn. Cl 2 Predict the products. Will a precipitate form? Yes. Sr. SO 4 is insoluble.

Double Replacement Reaction

Double Replacement Reaction
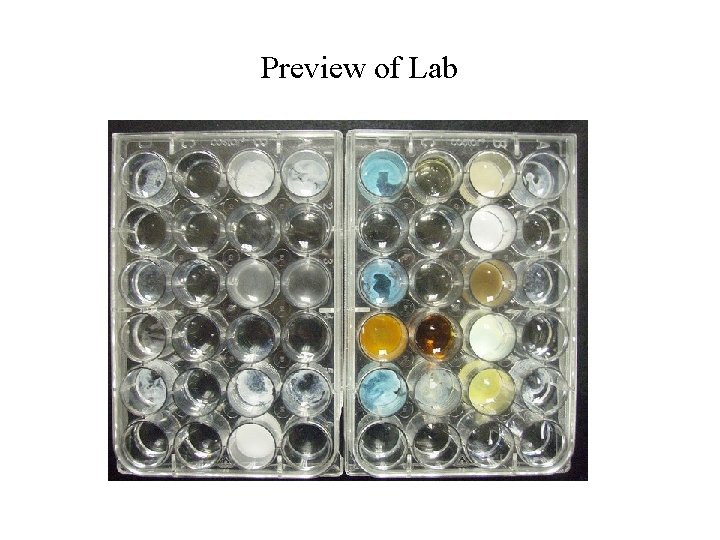
Preview of Lab
- Slides: 11