Predicting products single and double reactions Single replacement

Predicting products: single and double reactions
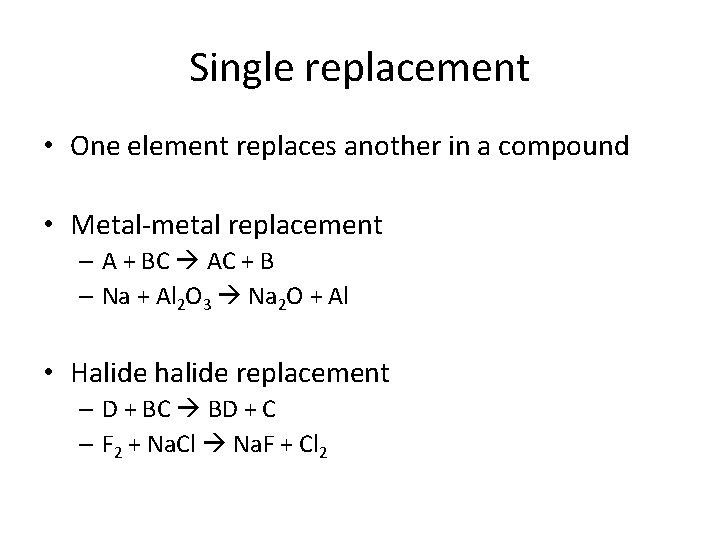
Single replacement • One element replaces another in a compound • Metal-metal replacement – A + BC AC + B – Na + Al 2 O 3 Na 2 O + Al • Halide halide replacement – D + BC BD + C – F 2 + Na. Cl Na. F + Cl 2
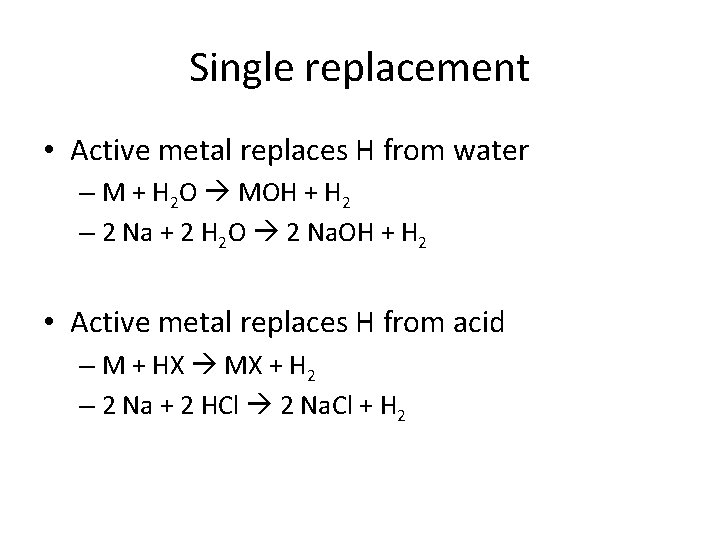
Single replacement • Active metal replaces H from water – M + H 2 O MOH + H 2 – 2 Na + 2 H 2 O 2 Na. OH + H 2 • Active metal replaces H from acid – M + HX MX + H 2 – 2 Na + 2 HCl 2 Na. Cl + H 2
Predicting products • Single replacement – depends on “activity” of the elements involved – Rx will only occur if single element is more reactive than element in compound
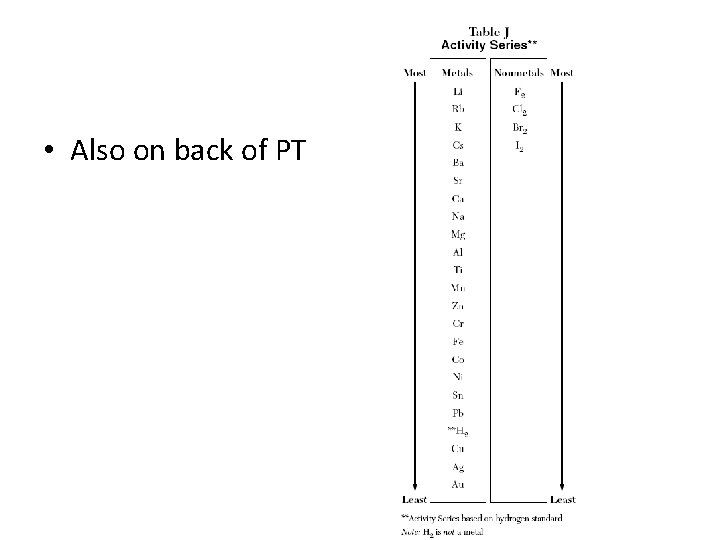
• Also on back of PT
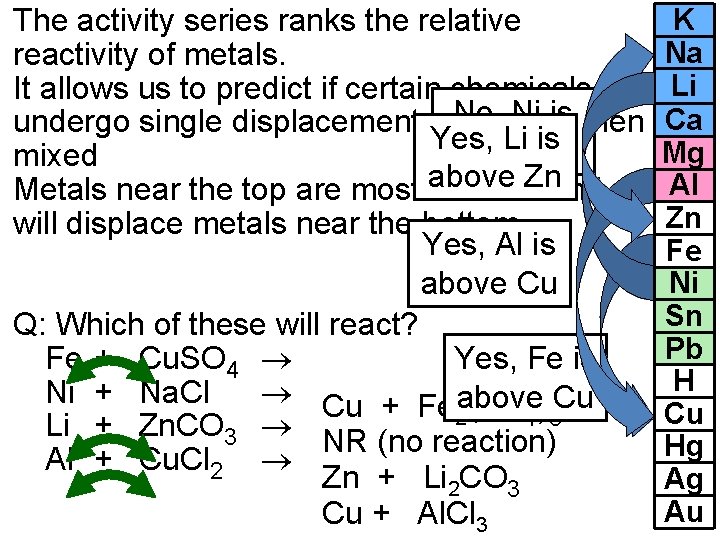
The activity series ranks the relative reactivity of metals. It allows us to predict if certain chemicals will No, Ni iswhen undergo single displacement reactions Yes, Li is below Na mixed Znand Metals near the top are most above reactive will displace metals near the bottom. Yes, Al is above Cu Q: Which of these will react? Fe + Cu. SO 4 Yes, Fe is Ni + Na. Cl Cu + Fe above Cu (SO ) 2 4 3 Li + Zn. CO 3 NR (no reaction) Al + Cu. Cl 2 Zn + Li 2 CO 3 Cu + Al. Cl 3 K Na Li Ca Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Au
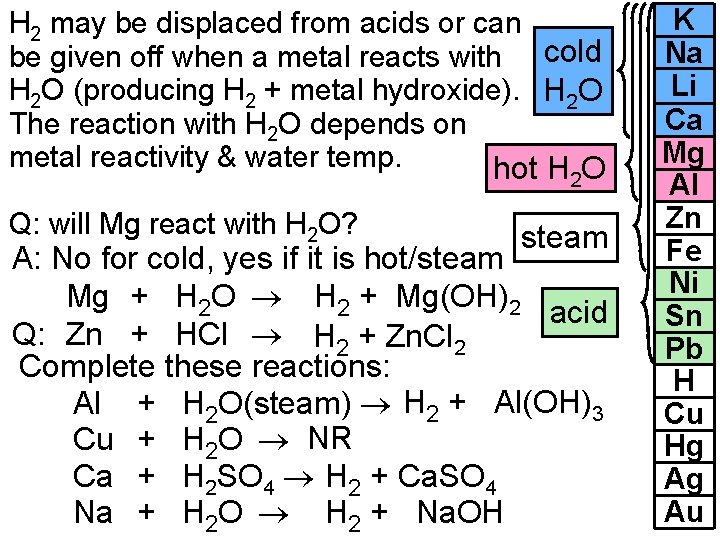
H 2 may be displaced from acids or can be given off when a metal reacts with cold H 2 O (producing H 2 + metal hydroxide). H 2 O The reaction with H 2 O depends on metal reactivity & water temp. hot H O 2 Q: will Mg react with H 2 O? steam A: No for cold, yes if it is hot/steam Mg + H 2 O H 2 + Mg(OH)2 acid Q: Zn + HCl H 2 + Zn. Cl 2 Complete these reactions: Al + H 2 O(steam) H 2 + Al(OH)3 Cu + H 2 O NR Ca + H 2 SO 4 H 2 + Ca. SO 4 Na + H 2 O H 2 + Na. OH K Na Li Ca Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Au
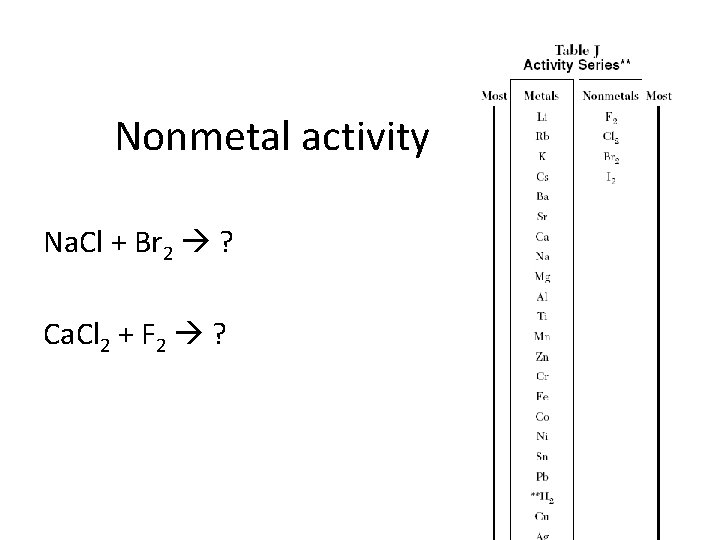
Nonmetal activity Na. Cl + Br 2 ? Ca. Cl 2 + F 2 ?
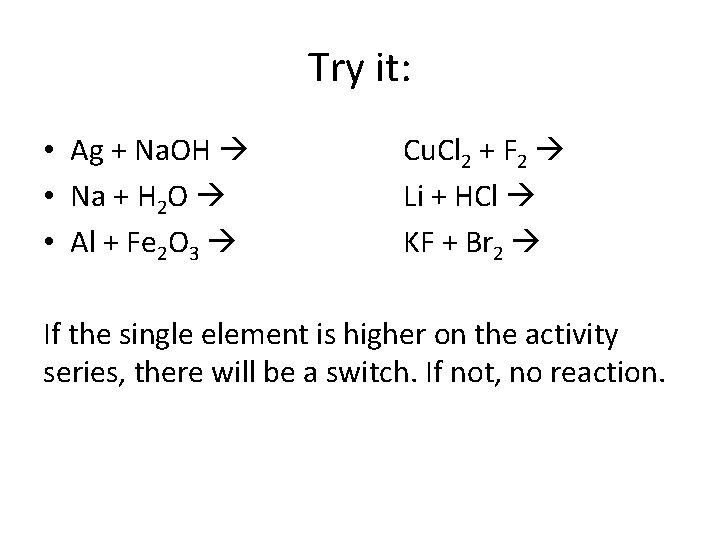
Try it: • Ag + Na. OH • Na + H 2 O • Al + Fe 2 O 3 Cu. Cl 2 + F 2 Li + HCl KF + Br 2 If the single element is higher on the activity series, there will be a switch. If not, no reaction.
Double Replacement • Two compounds exchange ions • AB + CD AD + CB • Formation of a precipitate – Ag. NO 3 + Na. Cl Ag. Cl (s) + Na. NO 3 • Acid-Base neutralization – Acid + base salt + water – HCl + Na. OH Na. Cl + H 2 O
Predicting products • Double replacement – depends on solubility of product –Predicting products’ solubility
Haha funny stuff. • https: //www. youtube. com/watch? v=slf 8 w 2 D HH 9 Y
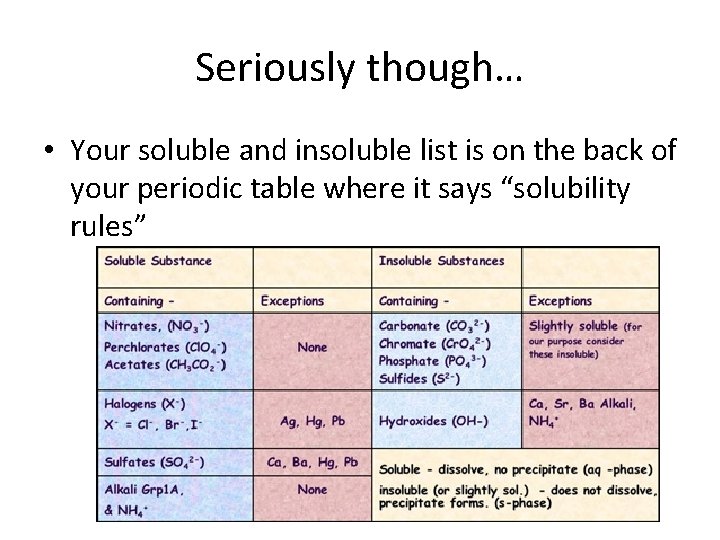
Seriously though… • Your soluble and insoluble list is on the back of your periodic table where it says “solubility rules”
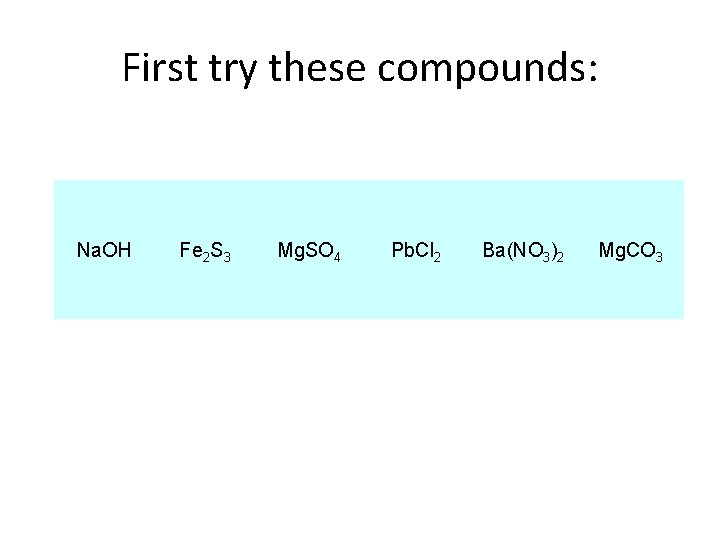
First try these compounds: Na. OH Fe 2 S 3 Mg. SO 4 Pb. Cl 2 Ba(NO 3)2 Mg. CO 3
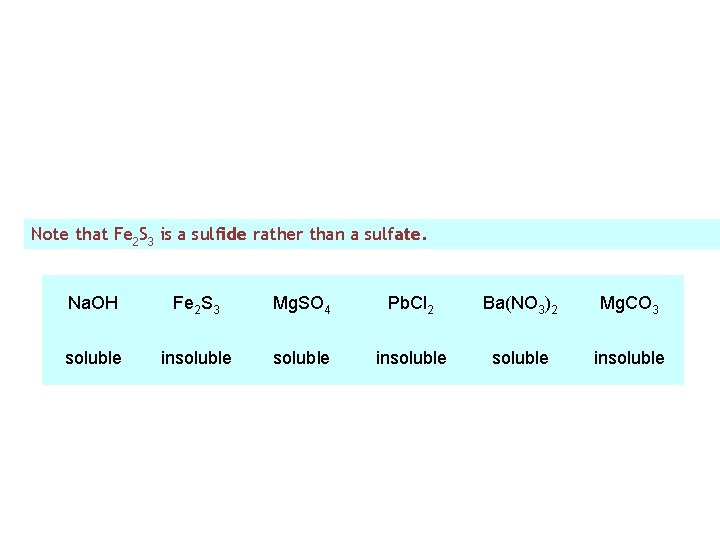
Note that Fe 2 S 3 is a sulfide rather than a sulfate. Na. OH Fe 2 S 3 Mg. SO 4 Pb. Cl 2 Ba(NO 3)2 Mg. CO 3 soluble insoluble
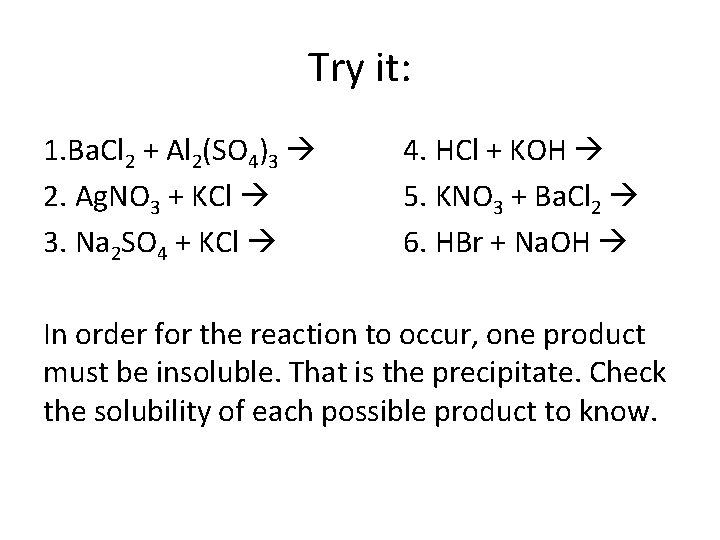
Try it: 1. Ba. Cl 2 + Al 2(SO 4)3 2. Ag. NO 3 + KCl 3. Na 2 SO 4 + KCl 4. HCl + KOH 5. KNO 3 + Ba. Cl 2 6. HBr + Na. OH In order for the reaction to occur, one product must be insoluble. That is the precipitate. Check the solubility of each possible product to know.
- Slides: 16