Predicting precipitation reactions Ionic reactions of two solutions





- Slides: 12

Predicting precipitation reactions

Ionic reactions of two solutions are double replacement reactions The ions switch partners in a double replacement reaction. barium chlorate + silver I sulfate barium sulfate + silver I chlorate Ba(Cl. O 3)2 (we + Ag 2 SO 4 Ba. SO 4 + Ag. Cl. O 3 are not balancing yet) Remember there will still be a cation with an anion. You can’t have 2 cations or anions together.

Example Calcium It Nitrate and Potassium Phosphate Ca(NO 3)2 + K 3 PO 4 ? helps to say the names of the ionic compounds Switch partners Calcium Phosphate and Potassium Nitrate What Ca 3(PO 4)2 + KNO 3 is the formula of these ionic compounds? Ca(NO 3)2 + K 3 PO 4 Ca 3(PO 4)2 + KNO 3

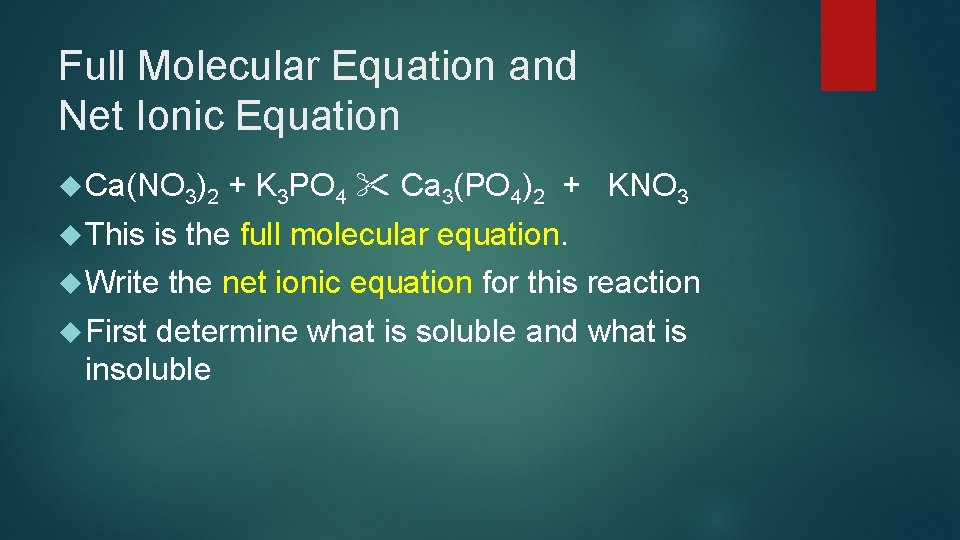
Full Molecular Equation and Net Ionic Equation Ca(NO 3)2 This is the full molecular equation. Write First + K 3 PO 4 Ca 3(PO 4)2 + KNO 3 the net ionic equation for this reaction determine what is soluble and what is insoluble

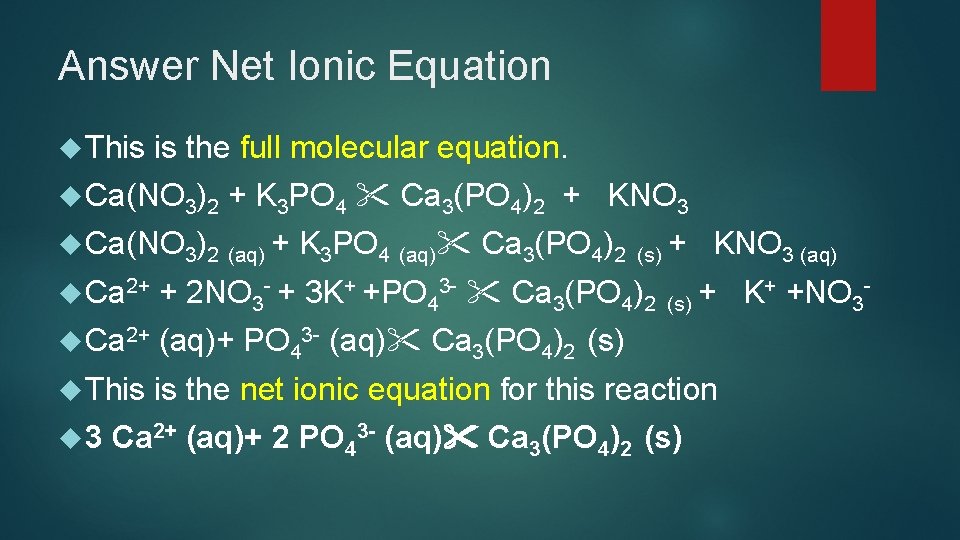
Answer Net Ionic Equation This is the full molecular equation. Ca(NO 3)2 + K 3 PO 4 Ca 3(PO 4)2 + KNO 3 Ca(NO 3)2 (aq) + K 3 PO 4 (aq) Ca 3(PO 4)2 (s) + KNO 3 (aq) Ca 2+ + 2 NO 3 - + 3 K+ +PO 43 - Ca 3(PO 4)2 Ca 2+ (aq)+ PO 43 - (aq) Ca 3(PO 4)2 (s) This is the net ionic equation for this reaction 3 + +NO + K (s) 3 Ca 2+ (aq)+ 2 PO 43 - (aq) Ca 3(PO 4)2 (s)

DO NOT!!!!! A common mistake is to try and carry the subscript over from the reactant side It helps to say the name of the compound because you have to write the appropriate ionic formula. Ca(NO 3)2 The + K 3 PO 4 Ca PO 4 + K 3(NO 3)2 charges don’t cancel out!!!!!

Predict the full molecular and net ionic equation between Copper II acetate and lithium sulfite Ammonium Aluminum oxalate and cobalt II chloride nitrate and sodium carbonate Hydrochloric acid and silver I nitrate

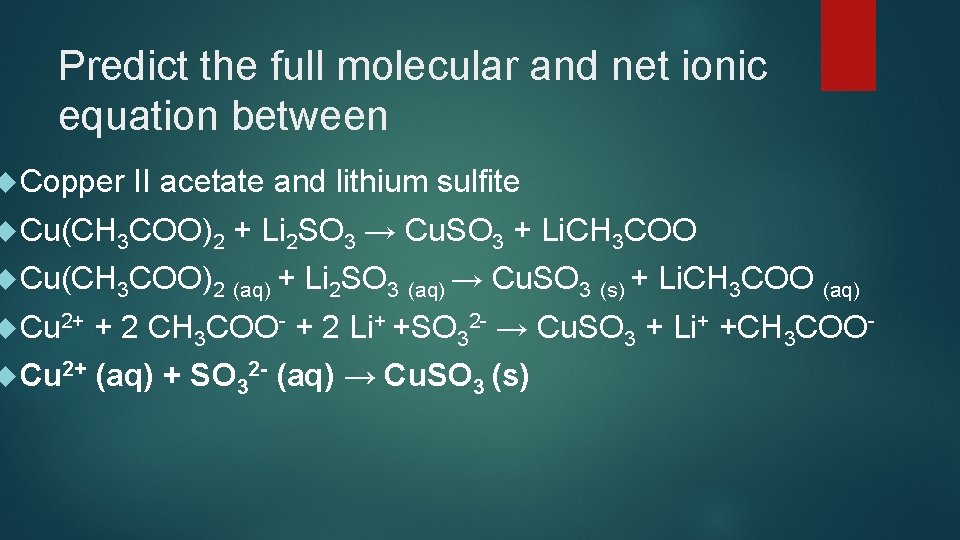
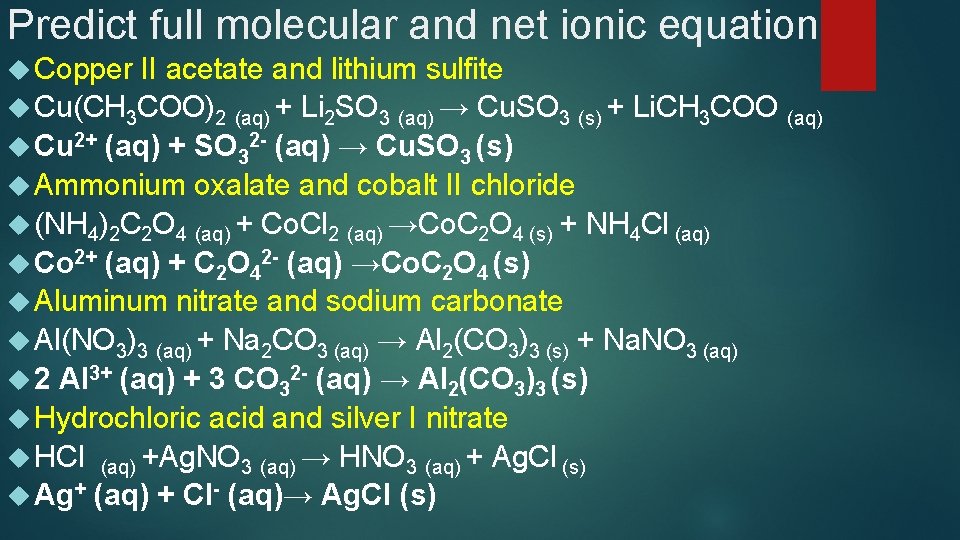
Predict the full molecular and net ionic equation between Copper II acetate and lithium sulfite Cu(CH 3 COO)2 + Li 2 SO 3 → Cu. SO 3 + Li. CH 3 COO Cu(CH 3 COO)2 (aq) + Li 2 SO 3 (aq) → Cu. SO 3 (s) + Li. CH 3 COO (aq) Cu 2+ + 2 CH 3 COO- + 2 Li+ +SO 32 - → Cu. SO 3 + Li+ +CH 3 COO- Cu 2+ (aq) + SO 32 - (aq) → Cu. SO 3 (s)

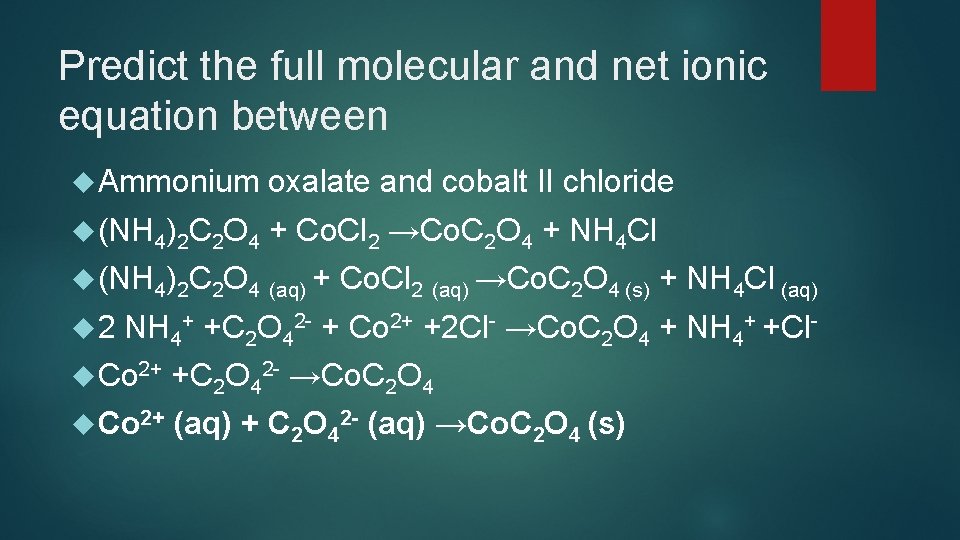
Predict the full molecular and net ionic equation between Ammonium oxalate and cobalt II chloride (NH 4)2 C 2 O 4 + Co. Cl 2 →Co. C 2 O 4 + NH 4 Cl (NH 4)2 C 2 O 4 (aq) + 2 Co. Cl 2 (aq) →Co. C 2 O 4 (s) + NH 4 Cl (aq) NH 4+ +C 2 O 42 - + Co 2+ +2 Cl- →Co. C 2 O 4 + NH 4+ +Cl- Co 2+ +C 2 O 42 - →Co. C 2 O 4 Co 2+ (aq) + C 2 O 42 - (aq) →Co. C 2 O 4 (s)

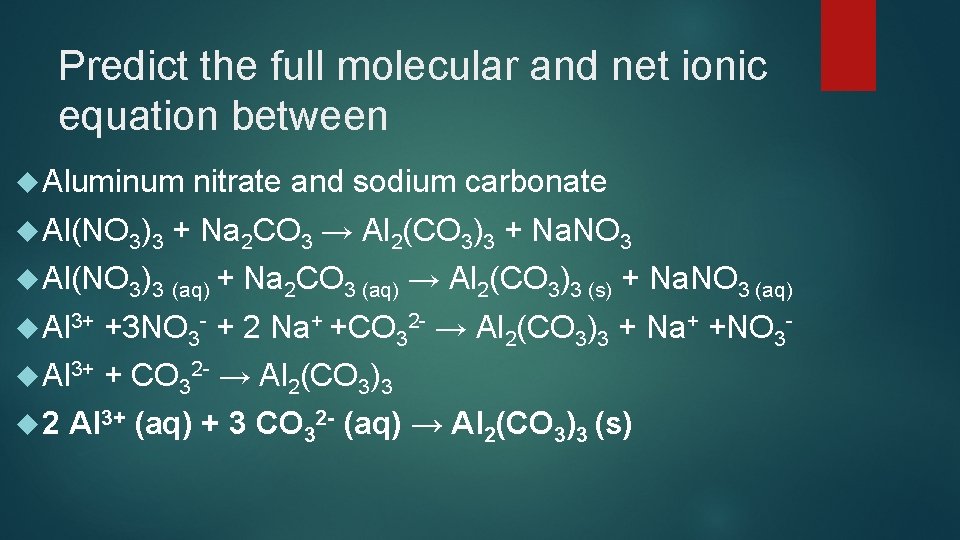
Predict the full molecular and net ionic equation between Aluminum Al(NO 3)3 nitrate and sodium carbonate + Na 2 CO 3 → Al 2(CO 3)3 + Na. NO 3 Al(NO 3)3 (aq) + Na 2 CO 3 (aq) → Al 2(CO 3)3 (s) + Na. NO 3 (aq) Al 3+ +3 NO 3 - + 2 Na+ +CO 32 - → Al 2(CO 3)3 + Na+ +NO 3 - Al 3+ + CO 32 - → Al 2(CO 3)3 2 Al 3+ (aq) + 3 CO 32 - (aq) → Al 2(CO 3)3 (s)

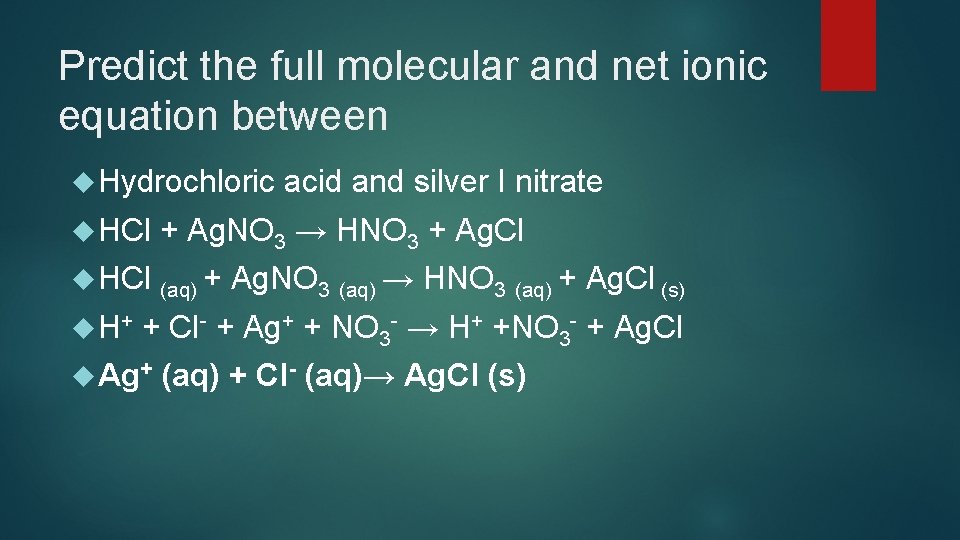
Predict the full molecular and net ionic equation between Hydrochloric HCl + Ag. NO 3 → HNO 3 + Ag. Cl HCl (aq) + H+ acid and silver I nitrate Ag. NO 3 (aq) → HNO 3 (aq) + Ag. Cl (s) + Cl- + Ag+ + NO 3 - → H+ +NO 3 - + Ag. Cl Ag+ (aq) + Cl- (aq)→ Ag. Cl (s)

Predict full molecular and net ionic equation Copper II acetate and lithium sulfite Cu(CH 3 COO)2 (aq) + Li 2 SO 3 (aq) → Cu. SO 3 (s) + Li. CH 3 COO (aq) Cu 2+ (aq) + SO 32 - (aq) → Cu. SO 3 (s) Ammonium oxalate and cobalt II chloride (NH 4)2 C 2 O 4 (aq) + Co. Cl 2 (aq) →Co. C 2 O 4 (s) + NH 4 Cl (aq) Co 2+ (aq) + C 2 O 42 - (aq) →Co. C 2 O 4 (s) Aluminum nitrate and sodium carbonate Al(NO 3)3 (aq) + Na 2 CO 3 (aq) → Al 2(CO 3)3 (s) + Na. NO 3 (aq) 2 Al 3+ (aq) + 3 CO 32 - (aq) → Al 2(CO 3)3 (s) Hydrochloric acid and silver I nitrate HCl (aq) +Ag. NO 3 (aq) → HNO 3 (aq) + Ag. Cl (s) Ag+ (aq) + Cl- (aq)→ Ag. Cl (s)