PRECLINICAL ASSESSMENT IN DRUG DEVELOPMENT PROCESS WHAT IS
















- Slides: 23

PRECLINICAL ASSESSMENT IN DRUG DEVELOPMENT PROCESS

WHAT IS DRUG DISCOVERY? • In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which drugs are discovered and/or designed. • In the past most drugs have been discovered either by identifying the active ingredient from traditional remedies or by serendipitous discovery. • A new approach has been to understand how disease and infection are controlled at the molecular and physiological level and to target specific entities based on this knowledge.

• The process of drug discovery involves the identification of candidates, synthesis, characterization, screening, and assays for therapeutic efficacy. • Drug discovery process include target selection, lead identification, and preclinical and clinical candidate selection. • Once a compound has shown its value in these tests, it will begin the process of drug development prior to clinical trials.


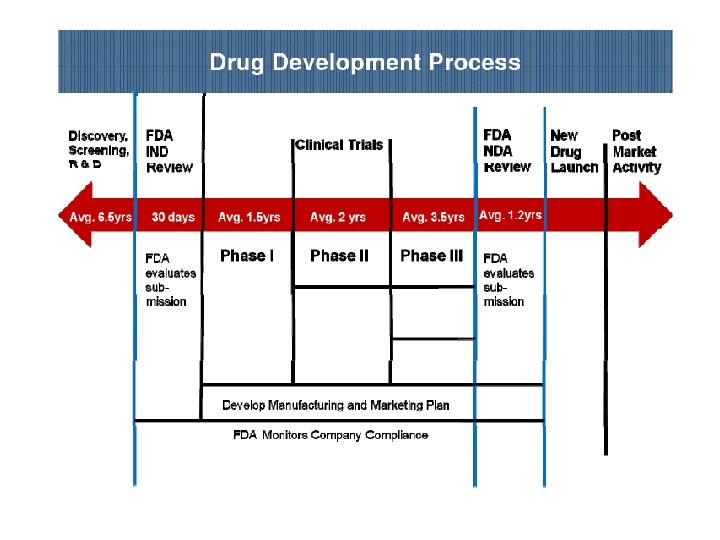
WHAT IS DRUG DEVELOPMENT ? • The various procedures and studies that must be undertaken to satisfy Drug Regulatory Authority requirements for drug approval and marketing. • This period includes preclinical testing of the candidate compounds, development and analysis of the dosage form, clinical studies, and submission of marketing approval documents.

• Drug development is a precarious pharmaceutical business with risks outweighing benefits. • Though risky, many major pharmaceutical companies are involved in drug development process, as it is essential for the survival of pharmaceutical companies and for the betterment of people with newer therapy for treating diseases.

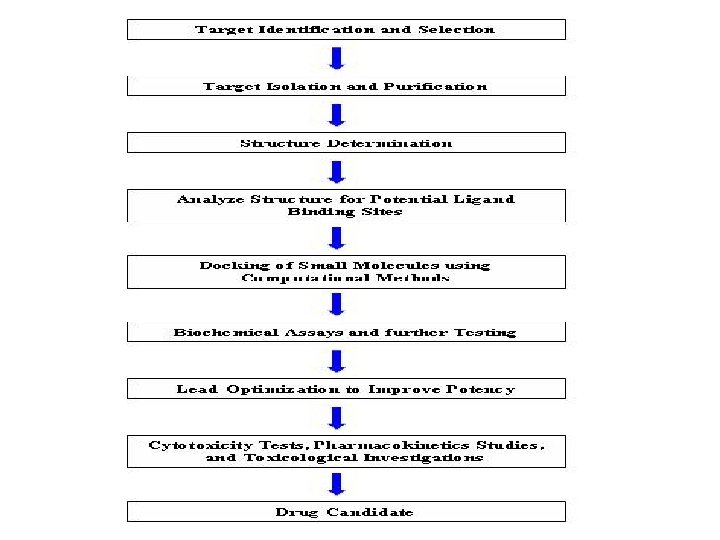
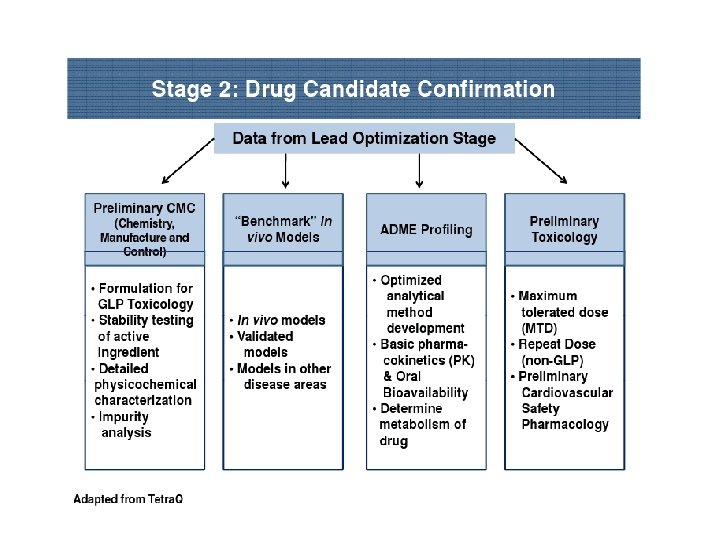
Involvement of different branches of Biological Sciences in New Drug Development

• Organic Chemistry involved in Synthesis & Purification • Instrumental Techniques for Product Characterization • Statistical approaches in Drug Discovery • Computer –Aided Drug Designs • Biotechnology revolutionized the Drug Discovery Process • Role of Pharmacology in Drug Development and Research • Toxicological examination to establish safety profile • Preclinical Pharmacokinetics


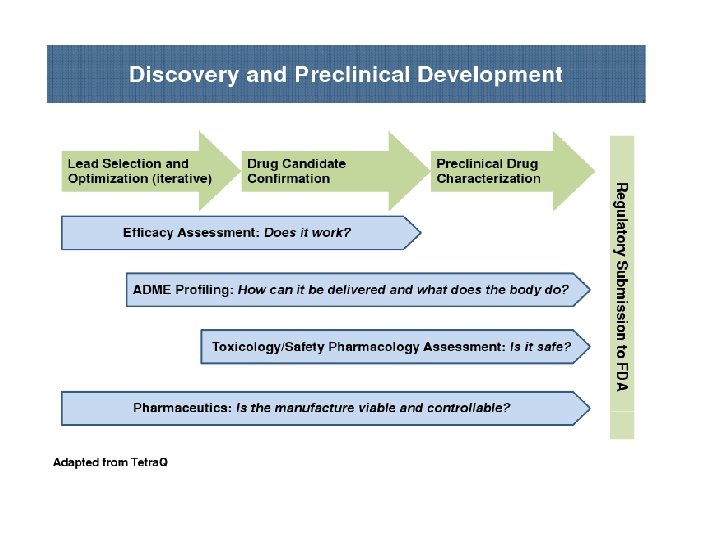
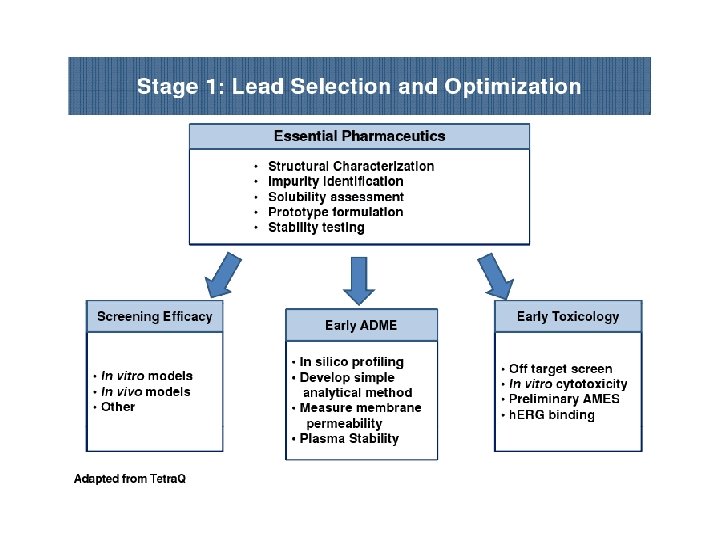
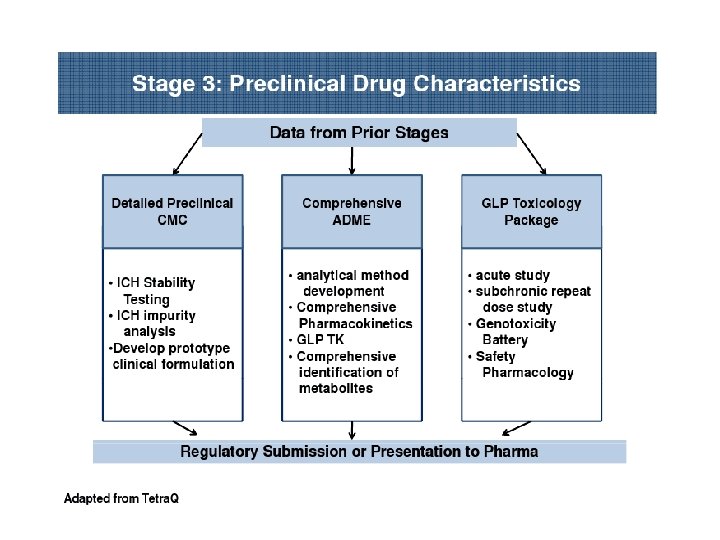
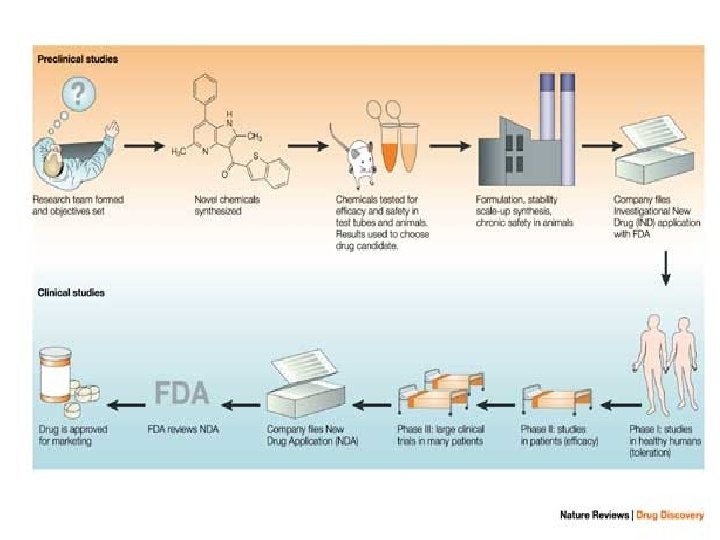
PRECLINICAL DEVELOPMENT • Pre-clinical development is a stage of research that begins before clinical trials (testing in humans) can begin, and during which important feasibility, iterative testing and safety (also known as Good Laboratory Practice or "GLP") data is collected. • The main goals of pre-clinical studies (also named preclinical studies and nonclinical studies) are to determine a product's ultimate safety profile. • Preclinical models are developed to test lead compounds for toxicity and efficacy. • They are valuable tools to minimize development costs and reduce failures prior to commencement of human trials.

Preclinical stage: • The goals of pre-clinical studies are to determine a drug’s pharmacodynamics, pharmacokinetics, ADME, and toxicity through animal testing. • This data allows researchers to all metrically estimate a safe starting dose of the drug for clinical trials in humans. • During preclinical drug development, the drug development team/company/ sponsor evaluates the drug's toxic and pharmacologic effects through in vitro and in vivo laboratory animal testing. • Genotoxicity screening is performed, as well as investigations on drug absorption and metabolism, the toxicity of the drug's metabolites, and the speed with which the drug and its metabolites are excreted from the body.

At the preclinical stage, the DRA will generally ask, at a minimum that sponsors: (1) Develop a pharmacological profile of the drug; (2) Determine the acute toxicity of the drug in at least two species of animals, and (3) Conduct short-term toxicity studies ranging from 2 weeks to 3 months, depending on the proposed duration of use of the substance in the proposed clinical studies.

Preclinical tests, therefore, are designed with the following considerations: (1) The expected duration of administration of the drug to human beings. (2) The age groups and physical status of the intended human subjects with special consideration for infants, pregnant women, or the aged. (3) The expected effects of the drug in humans.

Prior to the institution of clinical testing in humans, a Notice of Claimed Investigational Exemption for a New Drug must be filed with the Drug Regulatory Authority by the sponsor. This form is also referred to as an IND, or investigational new drug application.

The IND must feature the following information: 1. The best available descriptive name of the drug including to the extent known, the chemical name and structure of any new drug substance (a new chemical entity). 2. A complete list of components of the drug. 3. The quantitative composition of the drug. 4. The name and address of the supplier of any new drug substance if other than the sponsor (the person or firm submitting the IND) and a description of the preparation (chemical synthesis or other method of manufacture) of any new drug substance.

5. A statement of the methods, facilities, and controls used for the manufacture, processing, and packaging of the new drug. 6. A statement covering all information available to the sponsor derived from preclinical investigations and any clinical studies and experience with the drug. 7. Copies of labels for the drugs and informational material that will be supplied to investigators. This material must describe the preclinical studies with the drug and describe all relevant hazards, side effects, contraindications, and other information pertinent to use of the drug by the investigator.

8. A description of the scientific training and experience considered appropriate by the sponsor to qualify an investigator as a suitable expert to investigate the drug. 9. The names and curriculum vitae of all investigators. 10. An outline of the planned investigations of the drug in humans.





