Precipitation Reactions Dont write Chemical Equations Chemical Reaction















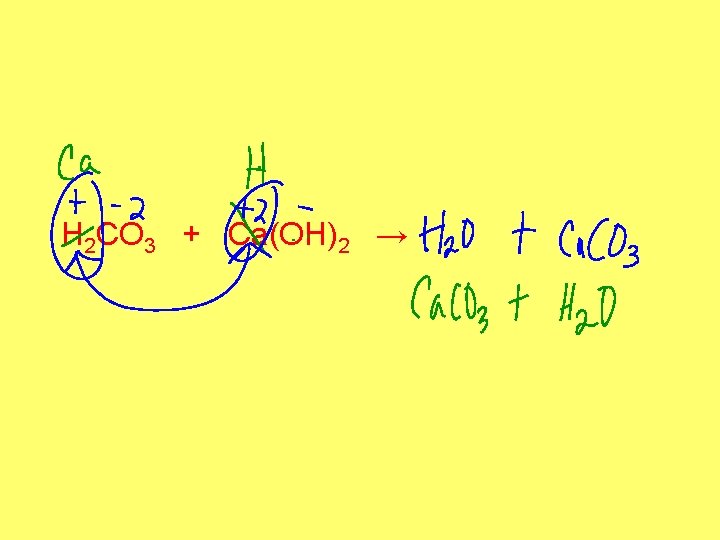








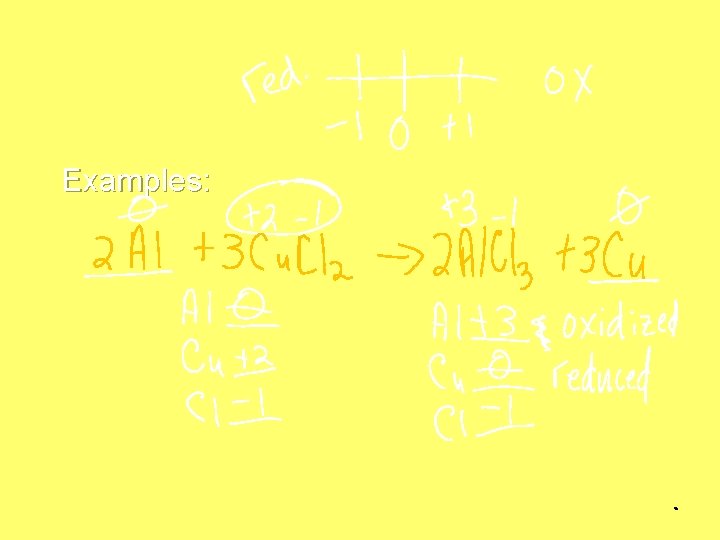

- Slides: 34

Precipitation Reactions

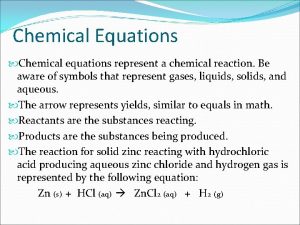
Don’t write - Chemical Equations • Chemical Reaction = new substance is produced. • • Reactants (R) – left of arrow Products (P) – right of arrow

Don’t Write - Indications of a Chemical Reaction • Evolution of heat/light • Exothermic (releases heat) • Endothermic (absorbs heat) • Production of a gas • Formation of a precipitate (solid) • Color change

Vocabulary to Know • Soluble – will dissolve (aq) • Insoluble – will not dissolve (s) Examples: Sand ? ? ? Sugar ? ? ? Salt ? ? ? Dirt ? ? ?

• Aqueous Solution – homogeneous mixture of a soluble compound dissolved in water • Precipitate – a solid formed in solution • Cation – positive ion (written first in a compound) • Anion – negative ion (written last in a compound)

Precipitation Reaction (also called): *Double Replacement *Ion switch Ions of 2 aqueous reactants exchange places and form 2 new products (need volunteers)

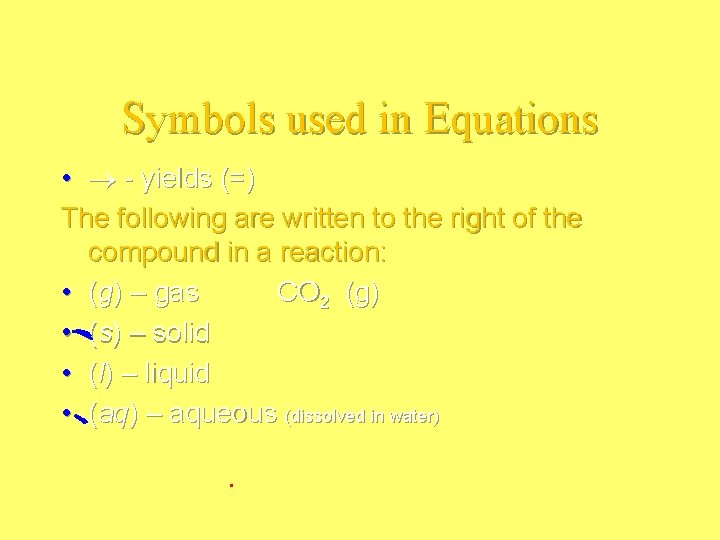
Symbols used in Equations • - yields (=) The following are written to the right of the compound in a reaction: • (g) – gas CO 2 (g) • (s) – solid • (l) – liquid • (aq) – aqueous (dissolved in water)

• One product will be soluble (aq) (dissolved in water – liquid). • One product will be insoluble (s) exist as a solid (it will precipitate out of solution)

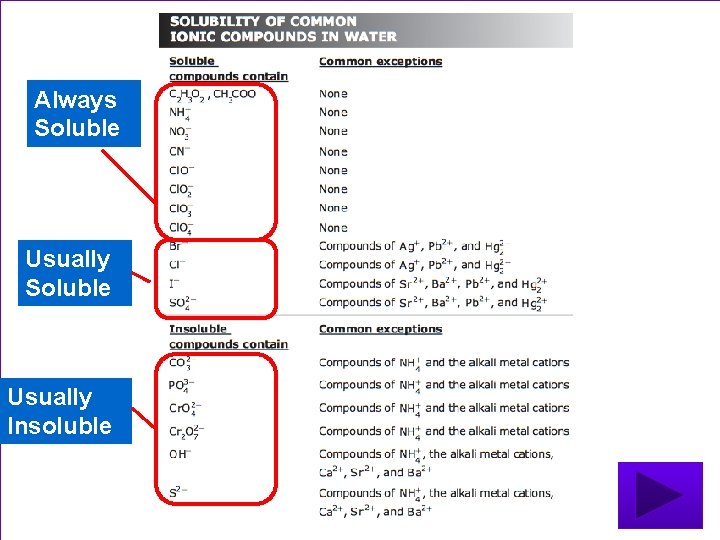
Soluble or Insoluble? ? • Check the solubility of common ionic compounds on reference materials sheet • Indicate which product is soluble and which is insoluble!!!!!

• Precipitation reactions - You. Tube • Double Replacement - Production of Precipitate • Orange tornado – You. Tube

• Are these compounds soluble or insoluble? (soluble =aq, insoluble = s) 1. K 2 SO 4 2. Ag. Cl 3. Ca. I 2 4. K 3 PO 4

Always Soluble Usually Insoluble

Precipitation examples 1. Zinc nitrate(aq) + ammonium sulfide(aq)→ ? 2. Calcium hydroxide(aq) + ammonium sulfate (aq)→

Acid-Base Reactions Neutralization

• Acid – Begins with hydrogen (H). *HCl *HNO 3 • Base – Has a hydroxide (OH) *Li. OH *Ca(OH)2 • Salt – An ionic compound *Na. Cl *NH 4 PO 4

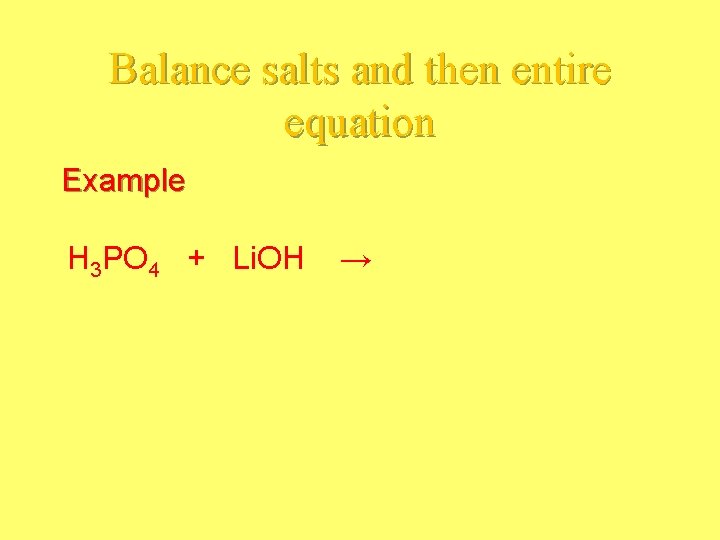
Acid Base Reaction • Acid and base reacts to form WATER and a SALT. - Balance the charges of the salt (+ comes from the base and neg comes from the acid)

Balance salts and then entire equation Example H 3 PO 4 + Li. OH →

• Fe(OH)2 + HBr →
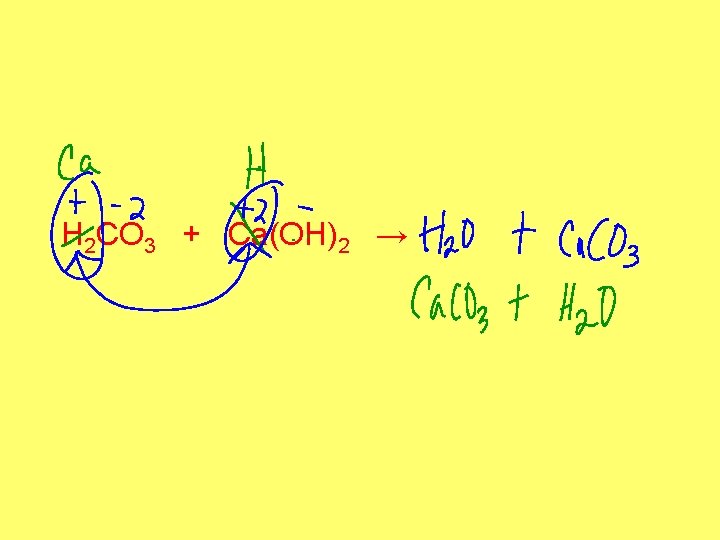
H 2 CO 3 + Ca(OH)2 →

What reaction will produce the salt K 2 SO 4

Reduction – Oxidation Reactions (Redox)

• Involves a transfer of electrons (gaining and losing). • Reaction will have a free element.

Example 2 Na + Cl 2 (Na is not gaining or losing e-) → 2 Na. Cl → (Na loses 1 e-) • This is a redox reaction because Sodium has to lose electrons to bond with chlorine. • And… chlorine gains electrons to bond with sodium.

Assigning oxidation numbers: 1. THE SUM OF OXIDATION NUMBERS (states) IN A COMPOUND WILL EQUAL ZERO. 2. Uncombined elements are 0 (zero). example: Cu O 2 S 8 3. Hydrogen and group I = + 1 4. Group II = +2 5. Oxygen = -2 6. Fluorine = -1



Examples:

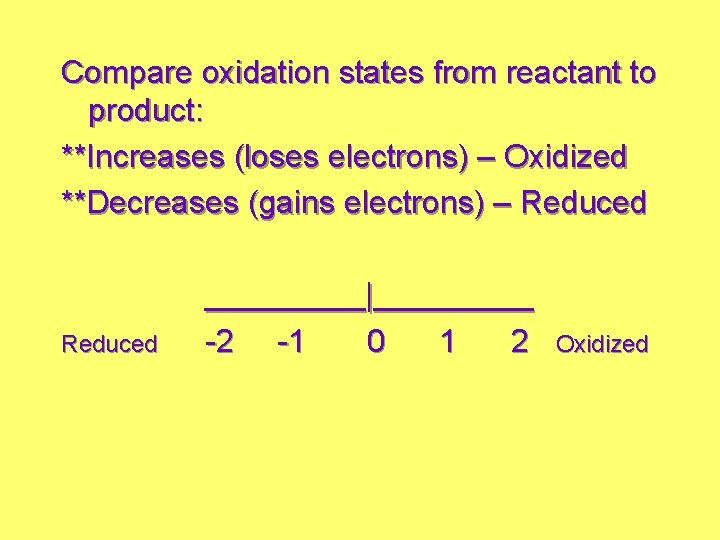
Compare oxidation states from reactant to product: **Increases (loses electrons) – Oxidized **Decreases (gains electrons) – Reduced _____|_____ -2 -1 0 1 2 Oxidized
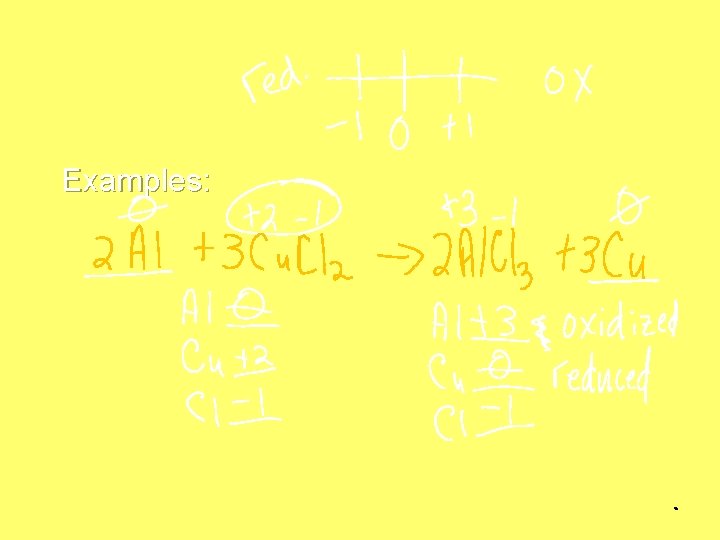
Examples:

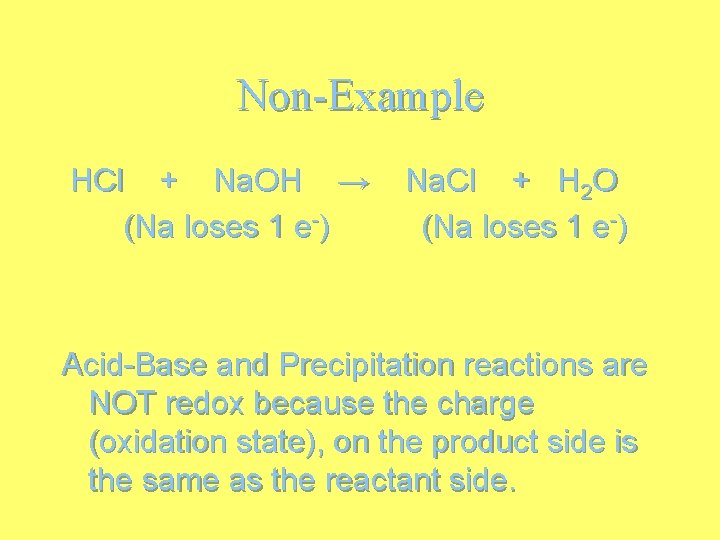
Non-Example HCl + Na. OH → (Na loses 1 e-) Na. Cl + H 2 O (Na loses 1 e-) Acid-Base and Precipitation reactions are NOT redox because the charge (oxidation state), on the product side is the same as the reactant side.

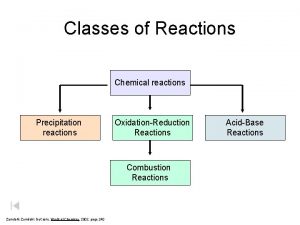
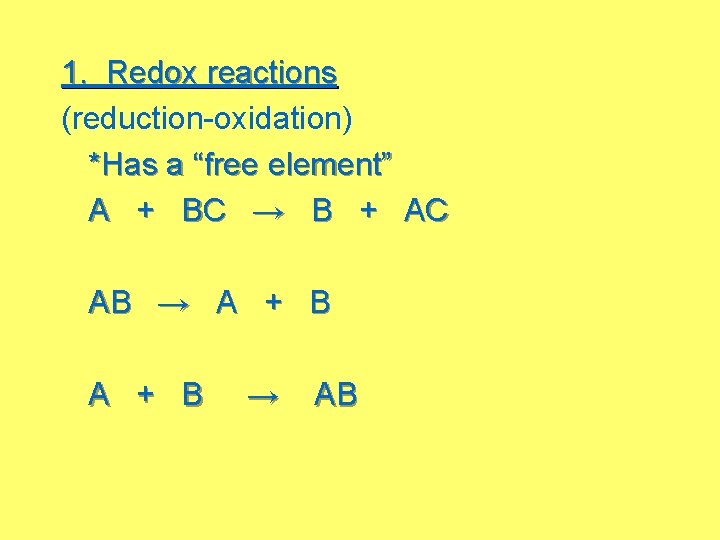
1. Redox reactions (reduction-oxidation) *Has a “free element” A + BC → B + AC AB → A + B → AB

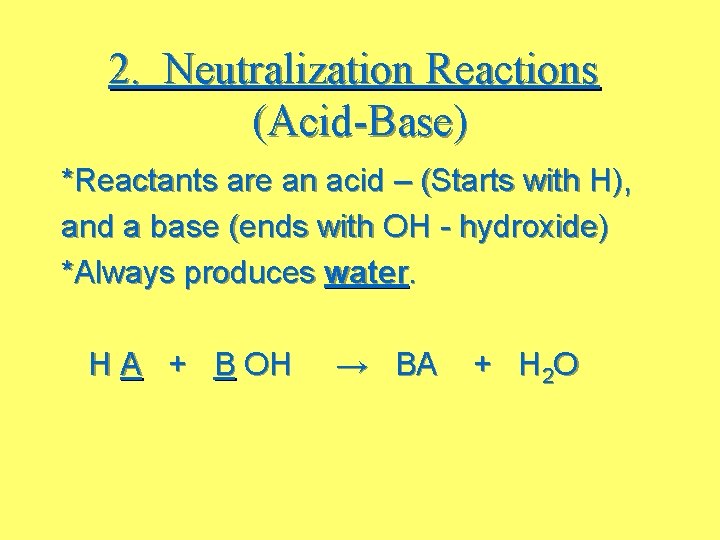
2. Neutralization Reactions (Acid-Base) *Reactants are an acid – (Starts with H), and a base (ends with OH - hydroxide) *Always produces water. H A + B OH → BA + H 2 O

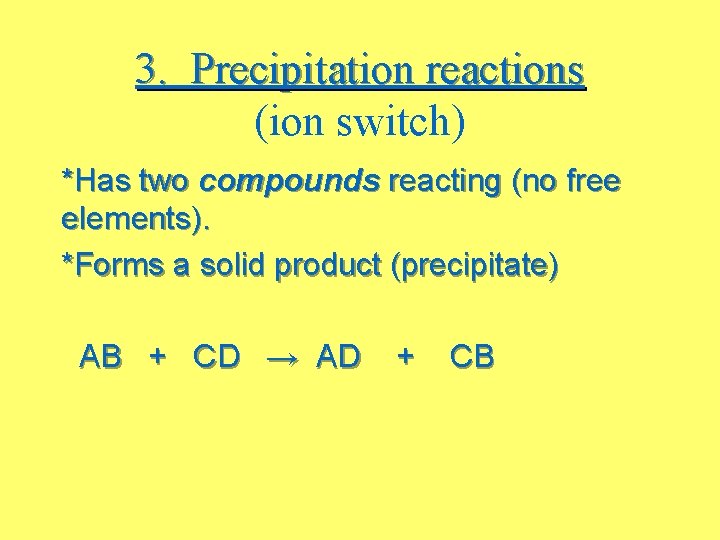
3. Precipitation reactions (ion switch) *Has two compounds reacting (no free elements). *Forms a solid product (precipitate) AB + CD → AD + CB

• Note to self – add reagents and assigning oxidation states and number line.
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Are kc and kp equal
Are kc and kp equal Dont ask dont tell political cartoon
Dont ask dont tell political cartoon Dont laugh at me dont call me names
Dont laugh at me dont call me names Types of reactions
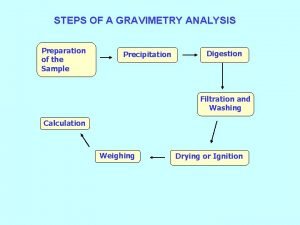
Types of reactions Gravimetry steps
Gravimetry steps Co precipitation and post precipitation
Co precipitation and post precipitation Toxic reactions chemical equations lesson 68 answers
Toxic reactions chemical equations lesson 68 answers Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Balancing chemical equations definition
Balancing chemical equations definition Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Synthesis reaction
Synthesis reaction Toxic reactions chemical equations
Toxic reactions chemical equations Lesson 90 solid evidence precipitation reactions
Lesson 90 solid evidence precipitation reactions Predicting precipitation reactions
Predicting precipitation reactions Double replacement precipitation
Double replacement precipitation Section 1 chemical changes
Section 1 chemical changes Half-life formula
Half-life formula How to identify a precipitation reaction
How to identify a precipitation reaction Precipitation word equation
Precipitation word equation Application of precipitation reaction
Application of precipitation reaction Soluble and insoluble salts
Soluble and insoluble salts Precipitation reaction
Precipitation reaction Half redox reaction
Half redox reaction Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Translate word equations to chemical equations
Translate word equations to chemical equations Oxidation half equations
Oxidation half equations Chapter 19 review oxidation-reduction reactions answers
Chapter 19 review oxidation-reduction reactions answers How to write half reactions
How to write half reactions How to write half reactions
How to write half reactions Stoichiometry mole island diagram
Stoichiometry mole island diagram Balancing redox reactions
Balancing redox reactions