Pradaxa Thats 19 years old in dog years













- Slides: 14

Pradaxa: That's 19 years old in dog years Sunday, November 22, 2020 Seth D Bilazarian, MD Dr. Seth@pmaonline. com

Disclosure • Nothing to disclose • I am a clinical investigator in several AF trials (RE-LY, ARISTOTLE, AVERROES, ENGAGE)

When will a generic version be available? • The first patent for Pradaxa is currently set to expire in February 2018. This is the earliest predictable date that a generic version could become available. • There are other circumstances that could come up to extend or shorten this exclusivity period. This could include such things as lawsuits or other patents for specific uses.

19 years in a dog's life According to www. pedigree. com (a calculator based on breed) at two years old a standard poodle or flat-coated retriever is the equivalent of a 19 -yearold human and is considered an adult. - Considered a senior dog at six years old (in human years, that's 47 years old)

Cardiomyogenesis in the aging and failing human heart Report that the human heart is characterized by a significant turnover of ventricular myocytes, endothelial cells, and fibroblasts, physiologically and pathologically. Renewal is very high shortly after birth, decreases during postnatal maturation, remains relatively constant in the adult organ, and increases dramatically with age. From 20 to 78 years of age, the adult human heart entirely replaces its myocyte, endothelial-cell, and fibroblast compartment eight, six, and eight times, respectively. Myocyte, endothelial-cell, and fibroblast regeneration is further enhanced with chronic heart failure. (Circulation 2012; 126: 1869– 1881)

All new agents compared with warfarin Advantages • No monitoring required • No variability • Fast onset of action • Fast offset • Lower intracranial hemorrhage rates (about 50% lower for all) Disadvantages • No reversibility • No monitoring • Expensive (higher tier by pharmacy benefit management) • Not once daily in AM • Less clinical experience • No data for cardiac issues other than nonvalvular AF

Anticoagulants Warfarin FOR: 1. Cheap 2. Long history Dabigatran FOR: 1. First mover 2. More effective Rivaroxaban FOR: 1. Once daily AGAINST: 1. Variability with food, drugs 2. Frequent monitoring AGAINST: 1. Not superior efficacy 2. Boxed warnings 3. Higher GI bleeding 4. Different doses 1. Higher MI rate 2. Higher GI bleeding

2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran) • Table 2. Recommendation for Emerging Antithrombotic Agents • 2011 focused update recommendation comments • Class I • Dabigatran is useful as an alternative to warfarin for the prevention of stroke and systemic thromboembolism in patients with paroxysmal to permanent AF and risk factors for stroke or systemic embolization who do not have a prosthetic heart valve or hemodynamically significant valve disease, severe renal failure (creatinine clearance 15 m. L/min), or advanced liver disease (impaired baseline clotting function). (Level of evidence: B) (Circulation 2011; 123: 1144– 1150)

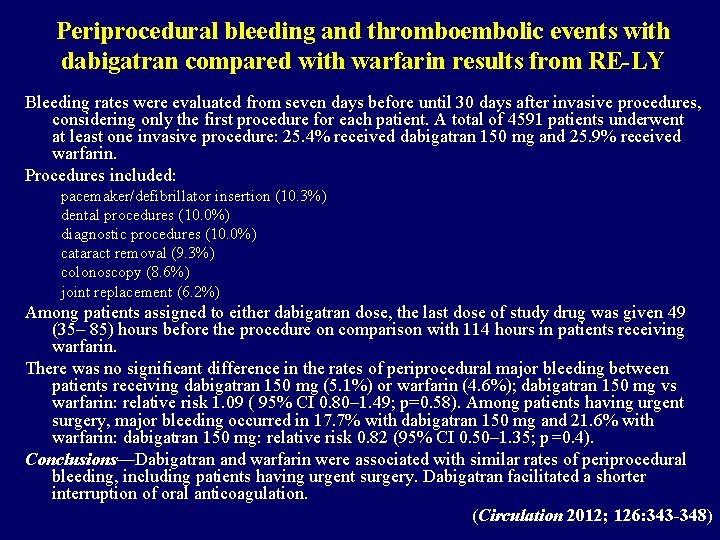
Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin results from RE-LY Bleeding rates were evaluated from seven days before until 30 days after invasive procedures, considering only the first procedure for each patient. A total of 4591 patients underwent at least one invasive procedure: 25. 4% received dabigatran 150 mg and 25. 9% received warfarin. Procedures included: pacemaker/defibrillator insertion (10. 3%) dental procedures (10. 0%) diagnostic procedures (10. 0%) cataract removal (9. 3%) colonoscopy (8. 6%) joint replacement (6. 2%) Among patients assigned to either dabigatran dose, the last dose of study drug was given 49 (35– 85) hours before the procedure on comparison with 114 hours in patients receiving warfarin. There was no significant difference in the rates of periprocedural major bleeding between patients receiving dabigatran 150 mg (5. 1%) or warfarin (4. 6%); dabigatran 150 mg vs warfarin: relative risk 1. 09 ( 95% CI 0. 80– 1. 49; p=0. 58). Among patients having urgent surgery, major bleeding occurred in 17. 7% with dabigatran 150 mg and 21. 6% with warfarin: dabigatran 150 mg: relative risk 0. 82 (95% CI 0. 50– 1. 35; p=0. 4). Conclusions—Dabigatran and warfarin were associated with similar rates of periprocedural bleeding, including patients having urgent surgery. Dabigatran facilitated a shorter interruption of oral anticoagulation. (Circulation 2012; 126: 343 -348)

Slow adoption •

Is the patient a good candidate for a new anticoagulant? (CRAB-I) • C = Good prescription coverage? • R = Normal renal function? • A = Are you an early adopter willing to take a new drug with one large trial in AF? • B = No history of GI bleeding? • I = For patients on warfarin, has there been INR instability requiring frequent dose changes?

Novel anticoagulants: Pradaxa/dabigatran adoption issues • Over 90% of cardiologists have used Pradaxa and only about 10% of internists • Some internists hesitant to use novel anticoagulants: novelty? Reversibility? Not understanding the new term nonvalvular AF*? • Issues with the elements of CRAB-I? – – Coverage hassles? GFR calculation hassles? Bleeding concerns? Instability? Someone else is managing the warfarin so may not even know • Bad drug commercials NVAF = No prosthetic heart valve or valvular disease that does not require surgical repair (RE-LY had 21% with valvular heart disease that met nonvalvular-AF criteria)

Conclusion • Pleased with adoption in my practice • Heartburn is a problem in some patients • Drug has superior efficacy, and I feel comfortable quoting that data to patients (FDA approved that in the label) • Evaluating renal function initially and at followup—I avoid it in GFR <40 • Clearly advantages for patient and practice reduced burden and ease of transition for surgery

 19 in dog years
19 in dog years Hepatita c
Hepatita c Thats just rude
Thats just rude Imagery in the great gatsby chapter 3
Imagery in the great gatsby chapter 3 Promotions o'cool
Promotions o'cool Thats normal
Thats normal What is the answer to life
What is the answer to life Daisy’s devine day
Daisy’s devine day Thats all im asking for
Thats all im asking for Uwb protocol analyzers
Uwb protocol analyzers Thats entertainment dance competition
Thats entertainment dance competition Todays objective
Todays objective Thats not fair
Thats not fair Pop thats their warmup
Pop thats their warmup Is this jane's dog yes it is....dog
Is this jane's dog yes it is....dog