Practice Inclass Test 2 for CHM 151 Instructor

- Slides: 5

Practice In-class Test 2 for CHM 151: Instructor, Ken Costello Equation Balancing, Classification, Prediction of Products, & Stoichiometry Change the blood test reading for calcium from conventional to world standard of mmol/L. 10 mg d. L 1 mol 40. 1 g Change the blood test reading 80 mg 1 mol for iron from conventional to d. L 55. 8 g molarity (M is mol/L). Change this blood test reading for cholesterol from conventional to % wt/vol d. L 100 m. L μ 2. 49 mmol L . 0143 mol milli 1 d. L 10 -9 = 0. 1 0. 001 = 80 mg Change this blood test reading 40 ng for Vitamin D from conventional m. L to ppb (μg/L) deci L 0. 001 = 0. 08 % wt/vol milli = 40 ppb nano 10 -6 0. 001 Sodium hydroxide (lye) can be used in closed environments to remove excess CO 2 gas. The balanced chemical equation is: 2 Na. OH(s) + CO 2(g) --> Na 2 CO 3(s) + H 2 O(g) Ace Hardware has these in one pound bottles. What mass of CO 2(g) will the bottle be able to absorb? Potassium nitrate decomposes on heating, producing potassium oxide and gaseous nitrogen and oxygen. 4 KNO 3(s) --> 2 K 2 O(s) + 2 N 2(g) + 5 O 2(g) We need to breath about 1 gram of oxygen (O 2) per minute. A pound of KNO 3 would provide how many minutes of oxygen?

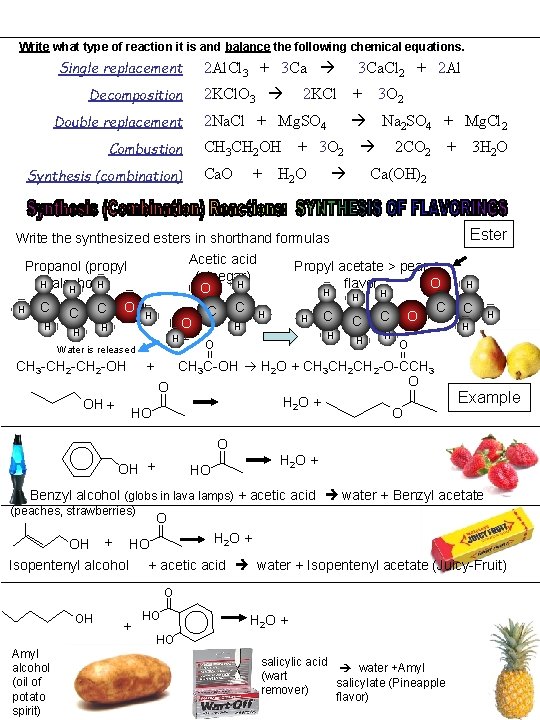
Write what type of reaction it is and balance the following chemical equations. Single replacement Decomposition Double replacement Combustion Synthesis (combination) 2 Al. Cl 3 + 3 Ca 2 KCl. O 3 2 KCl 2 Na. Cl + Mg. SO 4 3 Ca. Cl 2 + 2 Al + 3 O 2 Na 2 SO 4 + Mg. Cl 2 CH 3 CH 2 OH + 3 O 2 Ca. O + H 2 O 2 CO 2 + 3 H 2 O Ca(OH)2 Ester Write the synthesized esters in shorthand formulas Acetic acid (vinegar) H O C C H O H Propanol (propyl H alcohol)H H H C C H H O H H Water is released Propyl acetate > pear O flavor H H C H O || H H C C H H O C OH + HO C H H O || CH 3 -CH 2 -OH + CH 3 C-OH H 2 O + CH 3 CH 2 -O-CCH 3 O O || || H 2 O + OH + O HO O || H Example H 2 O + Benzyl alcohol (globs in lava lamps) + acetic acid water + Benzyl acetate (peaches, strawberries) OH + HO O || H 2 O + Isopentenyl alcohol + acetic acid water + Isopentenyl acetate (Juicy-Fruit) OH Amyl alcohol (oil of potato spirit) + HO O || H 2 O + HO salicylic acid water +Amyl (wart salicylate (Pineapple remover) flavor)

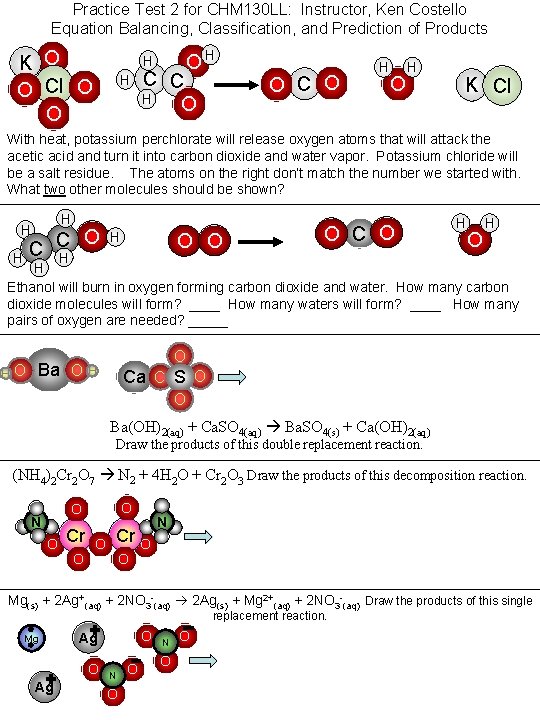
Practice Test 2 for CHM 130 LL: Instructor, Ken Costello Equation Balancing, Classification, and Prediction of Products K O O Cl O O OH H H C C H O O C O H K Cl With heat, potassium perchlorate will release oxygen atoms that will attack the acetic acid and turn it into carbon dioxide and water vapor. Potassium chloride will be a salt residue. The atoms on the right don’t match the number we started with. What two other molecules should be shown? H H H O C C H H O O O C O H H Ethanol will burn in oxygen forming carbon dioxide and water. How many carbon dioxide molecules will form? ____ How many waters will form? ____ How many pairs of oxygen are needed? _____ H O Ba O O H Ca O S O O Ba(OH)2(aq) + Ca. SO 4(aq) Ba. SO 4(s) + Ca(OH)2(aq) Draw the products of this double replacement reaction. (NH 4)2 Cr 2 O 7 N 2 + 4 H 2 O + Cr 2 O 3 Draw the products of this decomposition reaction. O O N O Cr O N O Mg(s) + 2 Ag+(aq) + 2 NO 3 -(aq) 2 Ag(s) + Mg 2+(aq) + 2 NO 3 -(aq) Draw the products of this single replacement reaction. Mg O Ag N O O

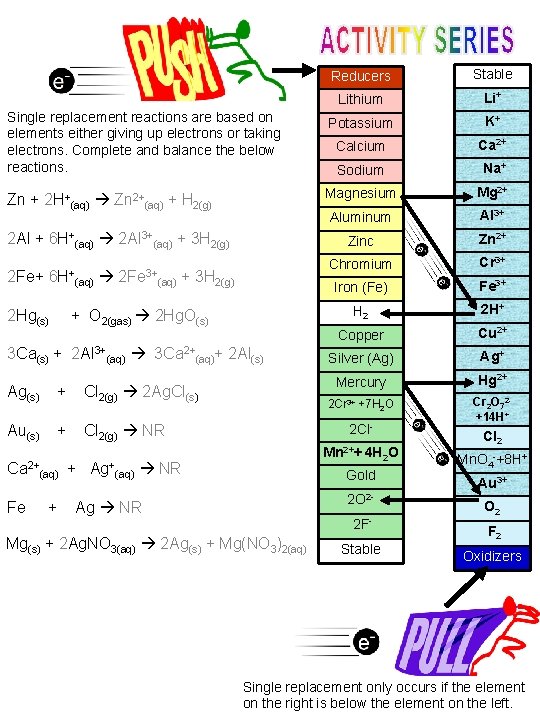
Reducers Stable Lithium Li+ Single replacement reactions are based on elements either giving up electrons or taking electrons. Complete and balance the below reactions. Potassium K+ Calcium Ca 2+ Sodium Na+ Zn + 2 H+(aq) Zn 2+(aq) + H 2(g) Magnesium Mg 2+ Aluminum Al 3+ Zinc Zn 2+ Chromium Cr 3+ Iron (Fe) Fe 3+ H 2 2 H+ Copper Cu 2+ Silver (Ag) Ag+ Mercury Hg 2+ 2 Cr 3+ +7 H 2 O Cr 2 O 72+14 H+ 2 Al + 6 H+(aq) 2 Al 3+(aq) + 3 H 2(g) 2 Fe+ 6 H+(aq) 2 Fe 3+(aq) + 3 H 2(g) 2 Hg(s) + O 2(gas) 2 Hg. O(s) 3 Ca(s) + 2 Al 3+(aq) 3 Ca 2+(aq)+ 2 Al(s) Ag(s) + Cl 2(g) 2 Ag. Cl(s) 2 Cl- Au(s) + Cl 2(g) NR Mn 2++ 4 H Ca 2+(aq) + Ag+(aq) NR Gold 2 O 2 - Fe + Ag NR 2 F- Mg(s) + 2 Ag. NO 3(aq) 2 Ag(s) + Mg(NO 3)2(aq) Stable 2 O Cl 2 Mn. O 4 -+8 H+ Au 3+ O 2 F 2 Oxidizers Single replacement only occurs if the element on the right is below the element on the left.

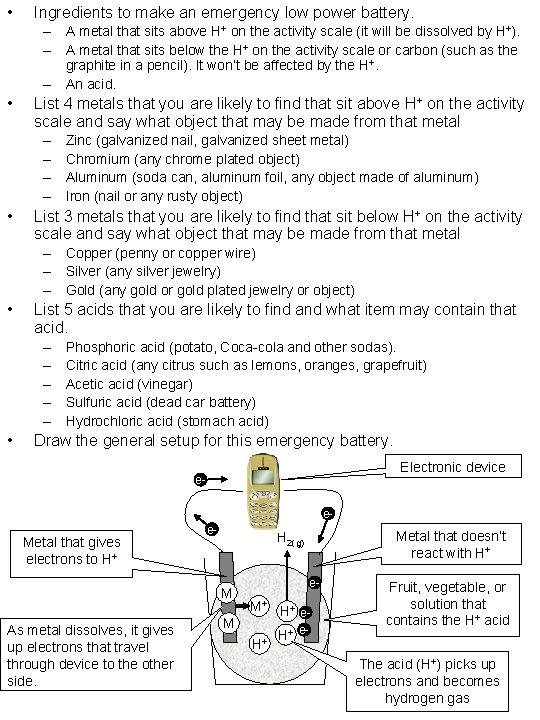
• Ingredients to make an emergency low power battery. – A metal that sits above H+ on the activity scale (it will be dissolved by H+). – A metal that sits below the H+ on the activity scale or carbon (such as the graphite in a pencil). It won’t be affected by the H+. – An acid. • List 4 metals that you are likely to find that sit above H+ on the activity scale and say what object that may be made from that metal – – • Zinc (galvanized nail, galvanized sheet metal) Chromium (any chrome plated object) Aluminum (soda can, aluminum foil, any object made of aluminum) Iron (nail or any rusty object) List 3 metals that you are likely to find that sit below H+ on the activity scale and say what object that may be made from that metal – Copper (penny or copper wire) – Silver (any silver jewelry) – Gold (any gold or gold plated jewelry or object) • List 5 acids that you are likely to find and what item may contain that acid. – – – • Phosphoric acid (potato, Coca-cola and other sodas). Citric acid (any citrus such as lemons, oranges, grapefruit) Acetic acid (vinegar) Sulfuric acid (dead car battery) Hydrochloric acid (stomach acid) Draw the general setup for this emergency battery. Electronic device ee- Metal that gives electrons to H+ e- M As metal dissolves, it gives up electrons that travel through device to the other side. Metal that doesn’t react with H+ H 2(g) M e- M+ H+ e. H+ H+ e- Fruit, vegetable, or solution that contains the H+ acid The acid (H+) picks up electrons and becomes hydrogen gas