Practical Organic Chemistry Lab No 1 Distillation Definition

Practical Organic Chemistry Lab No. 1 Distillation Definition , Principle , Uses , Types

Distillation: (Purification technique) is a process of separating the component or substances from a liquid mixture by evaporation and condensation or to purify an impure liquid. To separate a mixture of liquids, the liquid can be heated to force components, which have different boiling points. Although the term is most commonly applied to liquids, the reverse process can be used to separate gases by liquefying components using changes in temperature and/or pressure.

Principle of Distillation ? Distillation involves the conversion of a liquid to its vapor upon heating, then cooling the vapor by (Liebig Condenser) to condense and collecting it into the flask.
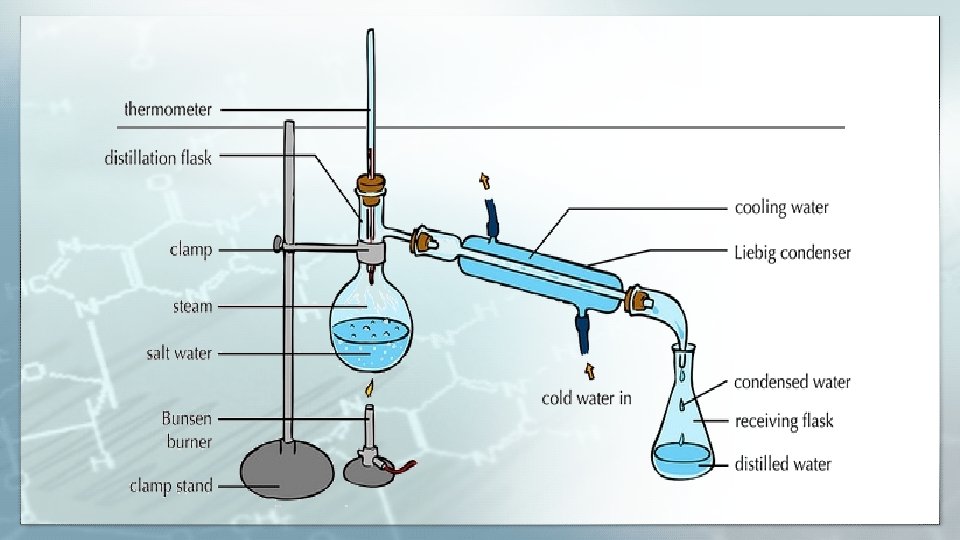

Uses Distillation The general use of Distillation is to purify the liquids which boils without decomposition and have non-volatile impurities Scientific Uses One practical use of distillation is in the laboratory, the process is used regularly in chemical and pharmaceutical research, quality assurance testing for many consumer products and law enforcement forensics.

1. Water Purification Water from natural sources contains a variety of minerals and other impurities, many of which can be removed upon heating to lose the basic (anion) or acidic (cation) part of the water, this process is done easily by distillation. Distilled water is commonly used in situations where the presence of minerals might reduce the effectiveness of certain equipment. 2. Perfume The aroma from various plants is contained (essential oils), which can be extracted through distillation.

3. Petroleum Products Several products can be produced from crude oil. Because each of these products has a unique boiling point, a process known as fractional distillation is used to refine oil into separate materials. 4. Food Flavoring Steam distillation is also used to create natural food flavorings. The most common are citrus oils and liquid extracts of various herbs and spices.
Types of Distillation § § Simple Distillation Fractional Distillation Steam Distillation Vacuum Distillation
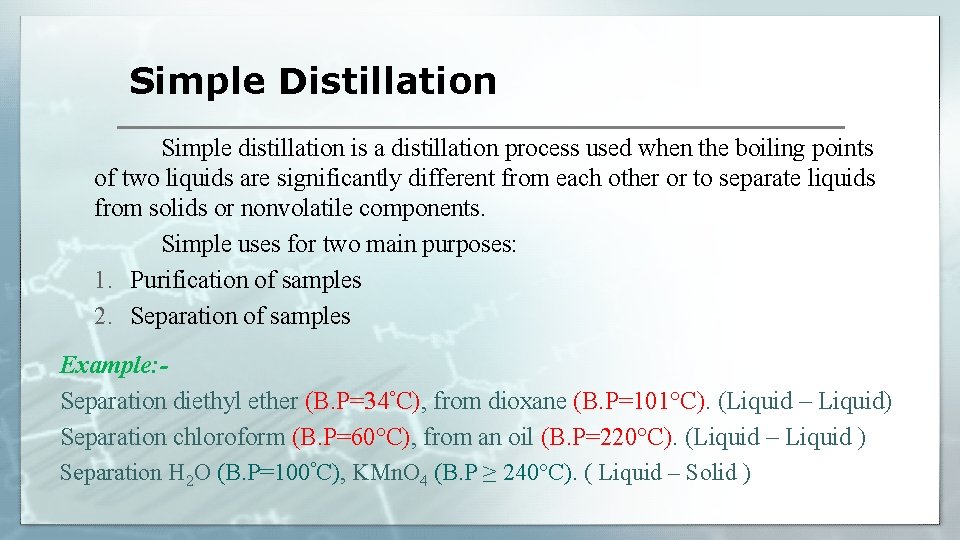
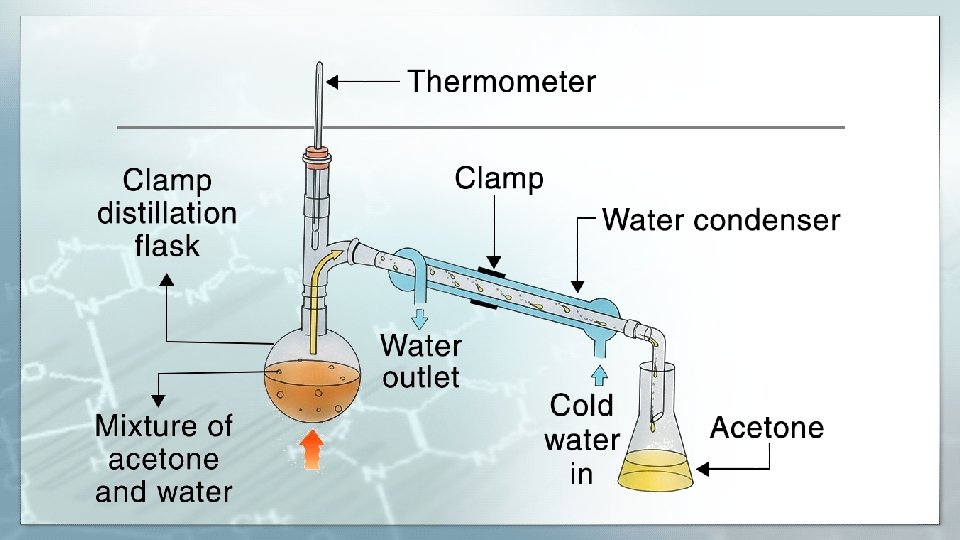
Simple Distillation Simple distillation is a distillation process used when the boiling points of two liquids are significantly different from each other or to separate liquids from solids or nonvolatile components. Simple uses for two main purposes: 1. Purification of samples 2. Separation of samples Example: Separation diethyl ether (B. P=34°C), from dioxane (B. P=101°C). (Liquid – Liquid) Separation chloroform (B. P=60°C), from an oil (B. P=220°C). (Liquid – Liquid ) Separation H 2 O (B. P=100°C), KMn. O 4 (B. P ≥ 240°C). ( Liquid – Solid )

Simple distillation can be used effectively to separate liquids that have at least 25 Co degrees difference in their boiling points. PROCEDURE: • As the liquid being distilled is heated, the vapors that form will be richest in the component of the mixture that boils at the lowest temperature. • Purified compounds will boil, and thus turn into vapors, over a relatively small temperature range (2 or 3°C); by carefully watching the temperature in the distillation flask, it is possible to affect a reasonably good separation • As distillation progresses, the concentration of the lowest boiling component will steadily decrease. • Eventually the temperature within the apparatus will begin to change; a pure compound is no longer being distilled. • The temperature will continue to increase until the boiling point of the next-lowest-boiling compound is approached. • When the temperature again stabilizes, another pure fraction of the distillate can be collected. • This fraction of distillate will be primarily the compound that boils at the second lowest temperature. • This process can be repeated until all the fractions of the original mixture have been separated.
- Slides: 11