Practical Guidance on Optimizing Management of Follicular Lymphoma






![Follicular Lymphoma: Pathology H&E of Lymph Node With Malignant Follicles[1] Nephron. Wikimedia Commons. H&E Follicular Lymphoma: Pathology H&E of Lymph Node With Malignant Follicles[1] Nephron. Wikimedia Commons. H&E](https://slidetodoc.com/presentation_image_h/0c8e0d12fcfbde4a8ffd2b11d8b94ff1/image-9.jpg)



![Sti. L NHL 1 -2003: BR vs R-CHOP in Newly Diagnosed FL PFS[1] Median Sti. L NHL 1 -2003: BR vs R-CHOP in Newly Diagnosed FL PFS[1] Median](https://slidetodoc.com/presentation_image_h/0c8e0d12fcfbde4a8ffd2b11d8b94ff1/image-29.jpg)




![SABRINA: Comparable Efficacy, Safety of Rituximab SQ vs IV + CHOP/CVP Response, n (%)[1] SABRINA: Comparable Efficacy, Safety of Rituximab SQ vs IV + CHOP/CVP Response, n (%)[1]](https://slidetodoc.com/presentation_image_h/0c8e0d12fcfbde4a8ffd2b11d8b94ff1/image-42.jpg)

![ASCT vs No ASCT for Early Progressing FL Study Casulo et al[1] Manna et ASCT vs No ASCT for Early Progressing FL Study Casulo et al[1] Manna et](https://slidetodoc.com/presentation_image_h/0c8e0d12fcfbde4a8ffd2b11d8b94ff1/image-47.jpg)




![PI 3 K Inhibitors: Efficacy Agent Idelalisib[1] Copanlisib[2] Duvelisib[3] DELTA CHRONOS‐ 1 DYNAMO II, PI 3 K Inhibitors: Efficacy Agent Idelalisib[1] Copanlisib[2] Duvelisib[3] DELTA CHRONOS‐ 1 DYNAMO II,](https://slidetodoc.com/presentation_image_h/0c8e0d12fcfbde4a8ffd2b11d8b94ff1/image-57.jpg)
![PI 3 K Inhibitors: Safety Agent Idelalisib[1, 2] Copanlisib[3, 4] Duvelisib[5, 6] delta alpha, PI 3 K Inhibitors: Safety Agent Idelalisib[1, 2] Copanlisib[3, 4] Duvelisib[5, 6] delta alpha,](https://slidetodoc.com/presentation_image_h/0c8e0d12fcfbde4a8ffd2b11d8b94ff1/image-58.jpg)





- Slides: 67

Practical Guidance on Optimizing Management of Follicular Lymphoma Supported by educational grants from Bayer Health. Care Pharmaceuticals Inc. , Celgene Corporation, and Epizyme Inc.

About These Slides § Please feel free to use, update, and share some or all of these slides in your noncommercial presentations to colleagues or patients § When using our slides, please retain the source attribution: Slide credit: clinicaloptions. com § These slides may not be published, posted online, or used in commercial presentations without permission. Please contact permissions@clinicaloptions. com for details

Program Faculty Jeff P. Sharman, MD Medical Director Hematology Research US Oncology Research Eugene, Oregon Emily Bucholtz, RPh Pharmacist Willamette Valley Cancer Institute Eugene, Oregon Nichole Fisher, RN, BSN Clinical Research Coordinator Registered Nurse, Lead Research, Oncology/Hematology Willamette Valley Cancer Institute Eugene, Oregon

Faculty Disclosures Jeff P. Sharman, MD, has disclosed that he has received funds for research support from Abb. Vie, Astra. Zeneca, Genentech, Gilead Sciences, Pharmacyclics, and TG Therapeutics and consulting fees from Abb. Vie, Astra. Zeneca, Genentech, Pharmacyclics, and TG Therapeutics. Nichole Fisher, RN, BSN, has disclosed that she has received consulting fees from Abb. Vie and Morpho. Sys, has received other financial or material support from Astra. Zeneca, and has ownership interest in TG Therapeutics. Emily Bucholtz, RPh, has no relevant conflicts of interest to report.

Agenda § Frontline Management of FL ‒ Background: Epidemiology, Grading, and Prognosis ‒ Data Informing Optimal Management of Newly Diagnosed FL § Sequencing of Therapies in R/R FL and Novel Approaches on the Horizon ‒ Data Informing Optimal Management of R/R FL ‒ Emerging Novel Agents and Strategies Under Investigation for R/R FL Slide credit: clinicaloptions. com

Frontline Management of Follicular Lymphoma

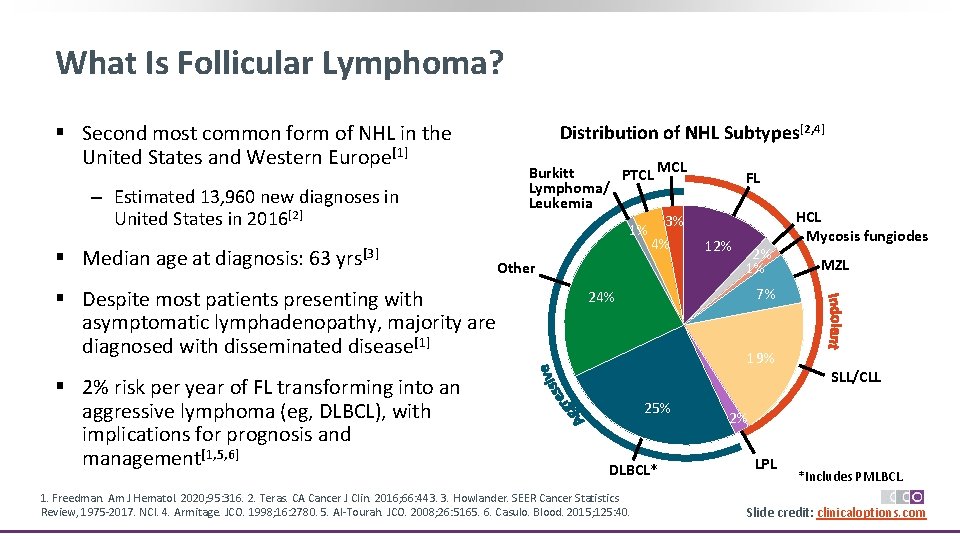
What Is Follicular Lymphoma? § Second most common form of NHL in the United States and Western Europe[1] ‒ Estimated 13, 960 new diagnoses in United States in 2016[2] § Median age at diagnosis: 63 yrs[3] Distribution of NHL Subtypes[2, 4] MCL Burkitt PTCL Lymphoma/ Leukemia 3% 1% 4% Other § Despite most patients presenting with asymptomatic lymphadenopathy, majority are diagnosed with disseminated disease[1] § 2% risk per year of FL transforming into an aggressive lymphoma (eg, DLBCL), with implications for prognosis and management[1, 5, 6] FL 12% 2% 1% MZL 7% 24% 19% 25% DLBCL* 1. Freedman. Am J Hematol. 2020; 95: 316. 2. Teras. CA Cancer J Clin. 2016; 66: 443. 3. Howlander. SEER Cancer Statistics Review, 1975‐ 2017. NCI. 4. Armitage. JCO. 1998; 16: 2780. 5. Al‐Tourah. JCO. 2008; 26: 5165. 6. Casulo. Blood. 2015; 125: 40. HCL Mycosis fungiodes SLL/CLL 2% LPL *Includes PMLBCL. Slide credit: clinicaloptions. com

Pathogenesis of Follicular Lymphoma Bone Marrow Lymph Node/Germinal Center t(14; 18) X ↑AID X Genetic Alteration t(14; 18)(q 32; q 21) Loss‐of‐function CREBBP mutations Mutations in MLL 2, EZH 2, MEF 2 B, etc. ; mutations in BCR signaling cascade: BTK, HVCN 1, SYK, etc. Functional Consequence ↑ BCL 2, ↑ AID, ↓ CREBBP Dysregulation of several genes Biological Effect Apoptosis‐resistant B‐cell Apoptosis‐resistant, somatic mutation‐prone B‐cell Proliferation, maturation arrest of Malignant progression; clonal apoptosis‐resistant, somatic selection, evolution, diversification mutation‐prone B‐cell Navarrete. Transl Cancer Res. 2017; 6(suppl 3): S 529. Slide credit: clinicaloptions. com
![Follicular Lymphoma Pathology HE of Lymph Node With Malignant Follicles1 Nephron Wikimedia Commons HE Follicular Lymphoma: Pathology H&E of Lymph Node With Malignant Follicles[1] Nephron. Wikimedia Commons. H&E](https://slidetodoc.com/presentation_image_h/0c8e0d12fcfbde4a8ffd2b11d8b94ff1/image-9.jpg)
Follicular Lymphoma: Pathology H&E of Lymph Node With Malignant Follicles[1] Nephron. Wikimedia Commons. H&E micrograph used in its original form under the terms and conditions of the Creative Commons Attribution‐Share. Alike 3. 0 Unported license (CC BY‐SA 3. 0: https: //creativecommons. org/licenses/by‐sa/3. 0/deed. en). Slide credit: clinicaloptions. com

Improved Prognosis for Patients With FL in New Treatment Era § FL typically regarded as chronic disease with good responses to initial therapy, but with eventual relapses to subsequent therapy[1, 2] § Recent therapeutic advances, most notably rituximab, have improved disease control and long‐term clinical outcomes[3‐ 5] ‒ Median survival is ~ 20 yrs, similar to age matched controls[5‐ 8] § Current goal of treatment: maintain best Qo. L by delaying disease progression—will this translate into an OS benefit with longer follow‐up? 1. 0 Presentation Yr 60 -Mo OS, % 120 -Mo OS, % 1995 -2004 82. 7 72. 3 0. 6 1985 -1994 70. 6 49. 6 1975 -1984 63. 5 41. 0 0. 4 1965 -1974 54. 1 31. 9 1955 -1964 41. 6 23. 2 1944 -1954 29. 3 17. 2 0. 8 OS (%) ‒ 10‐yr survival rate: 64% to 92%[3] OS Improvement in Indolent B-Cell Lymphoma from 1944 to 2004: the MDACC Experience[9] 0. 2 0 0 12 24 36 48 60 72 84 96 108 120 Mos 1. WHO. Follicular lymphoma. 2014. 2. ACS. Treating B‐cell non‐Hodgkin lymphoma. 3. Freedman. Am J Hematol. 2020; 95: 316. 4. Kahl. Blood. 2016; 127: 2055. 5. Provencio. PLo. S ONE. 2017; 12: e 0177204. 6. Maurer. Am J Hematol. 2016; 91: 1096. 7. Swenson. JCO. 2005; 23: 5019. 8. Tan. Blood. 2013; 122: 981. 9. Neelapu. 60 Years of Survival Outcomes at the MD Anderson Cancer Center. New York, NY: Springer; 2013. p. 241. Slide credit: clinicaloptions. com

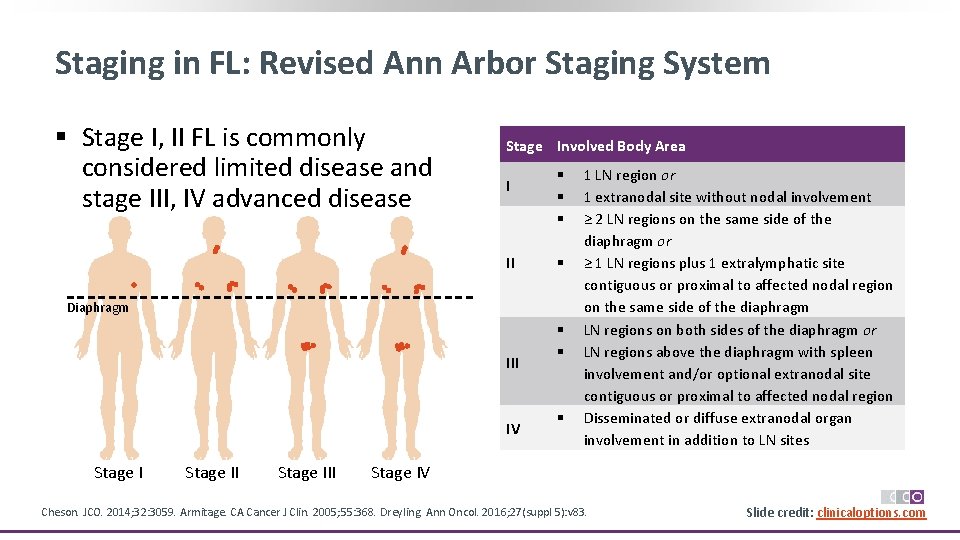
Staging in FL: Revised Ann Arbor Staging System § Stage I, II FL is commonly considered limited disease and stage III, IV advanced disease Stage Involved Body Area I II § § Diaphragm III IV Stage III § § § 1 LN region or 1 extranodal site without nodal involvement ≥ 2 LN regions on the same side of the diaphragm or ≥ 1 LN regions plus 1 extralymphatic site contiguous or proximal to affected nodal region on the same side of the diaphragm LN regions on both sides of the diaphragm or LN regions above the diaphragm with spleen involvement and/or optional extranodal site contiguous or proximal to affected nodal region Disseminated or diffuse extranodal organ involvement in addition to LN sites Stage IV Cheson. JCO. 2014; 32: 3059. Armitage. CA Cancer J Clin. 2005; 55: 368. Dreyling. Ann Oncol. 2016; 27(suppl 5): v 83. Slide credit: clinicaloptions. com

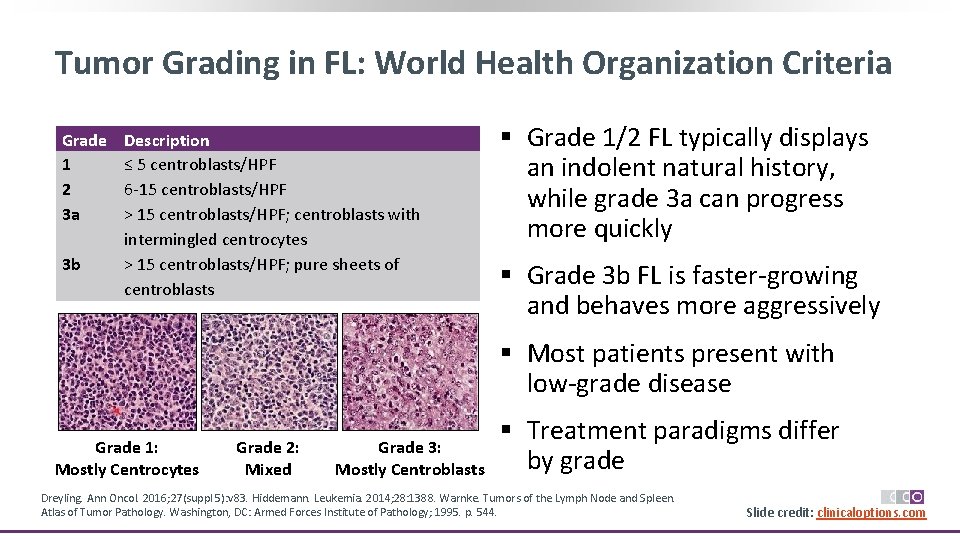
Tumor Grading in FL: World Health Organization Criteria Grade 1 2 3 a 3 b Description ≤ 5 centroblasts/HPF 6‐ 15 centroblasts/HPF > 15 centroblasts/HPF; centroblasts with intermingled centrocytes > 15 centroblasts/HPF; pure sheets of centroblasts § Grade 1/2 FL typically displays an indolent natural history, while grade 3 a can progress more quickly § Grade 3 b FL is faster‐growing and behaves more aggressively § Most patients present with low‐grade disease Grade 1: Mostly Centrocytes Grade 2: Mixed Grade 3: Mostly Centroblasts § Treatment paradigms differ by grade Dreyling. Ann Oncol. 2016; 27(suppl 5): v 83. Hiddemann. Leukemia. 2014; 28: 1388. Warnke. Tumors of the Lymph Node and Spleen. Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 1995. p. 544. Slide credit: clinicaloptions. com

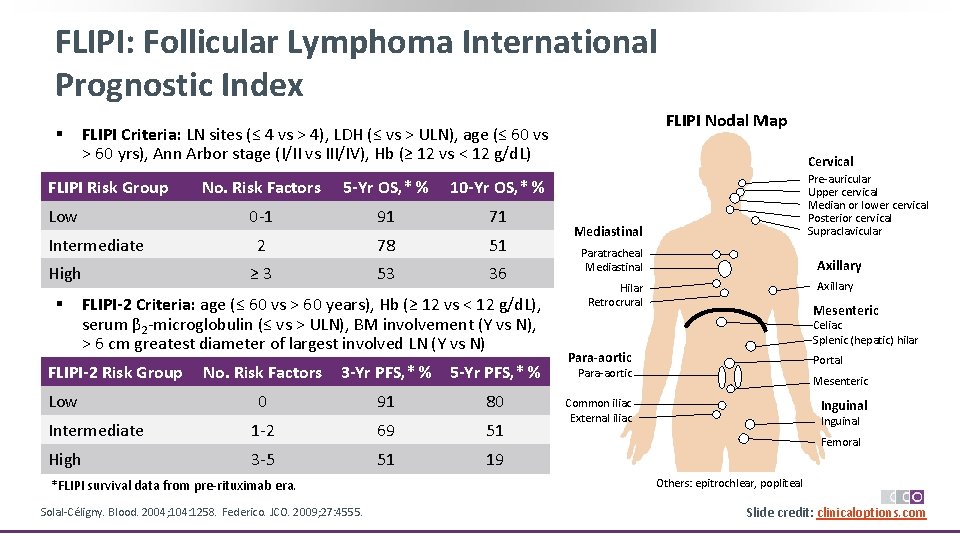
FLIPI: Follicular Lymphoma International Prognostic Index § FLIPI Risk Group Low Intermediate High § FLIPI Nodal Map FLIPI Criteria: LN sites (≤ 4 vs > 4), LDH (≤ vs > ULN), age (≤ 60 vs > 60 yrs), Ann Arbor stage (I/II vs III/IV), Hb (≥ 12 vs < 12 g/d. L) No. Risk Factors 5 -Yr OS, * % 10 -Yr OS, * % 0‐ 1 91 71 2 78 51 ≥ 3 53 36 FLIPI-2 Criteria: age (≤ 60 vs > 60 years), Hb (≥ 12 vs < 12 g/d. L), serum β 2‐microglobulin (≤ vs > ULN), BM involvement (Y vs N), > 6 cm greatest diameter of largest involved LN (Y vs N) FLIPI-2 Risk Group Low No. Risk Factors 3 -Yr PFS, * % 5 -Yr PFS, * % 0 91 80 Intermediate 1‐ 2 69 51 High 3‐ 5 51 19 *FLIPI survival data from pre‐rituximab era. Solal‐Céligny. Blood. 2004; 104: 1258. Federico. JCO. 2009; 27: 4555. Cervical Pre‐auricular Upper cervical Median or lower cervical Posterior cervical Supraclavicular Mediastinal Paratracheal Mediastinal Axillary Hilar Retrocrural Mesenteric Celiac Splenic (hepatic) hilar Para-aortic Portal Para‐aortic Mesenteric Common iliac External iliac Inguinal Femoral Others: epitrochlear, popliteal Slide credit: clinicaloptions. com

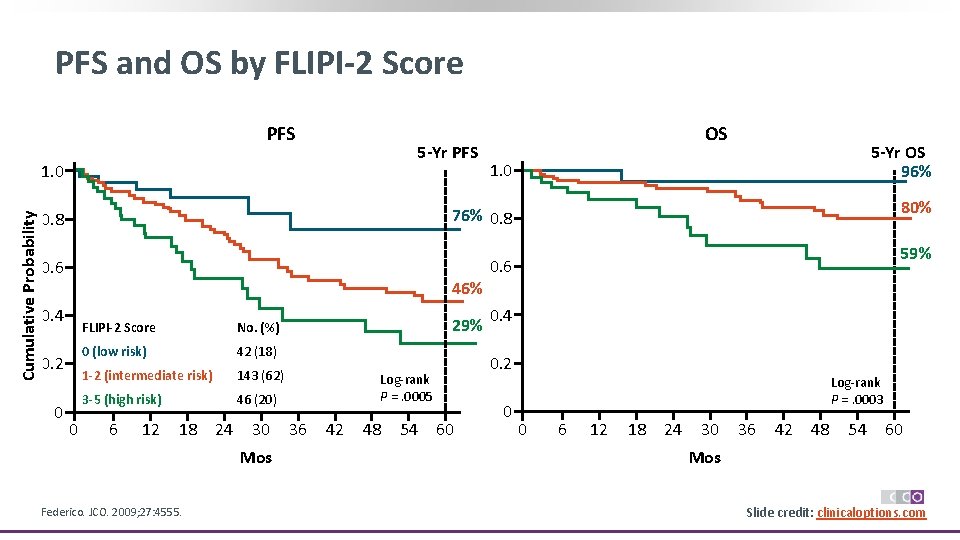
PFS and OS by FLIPI-2 Score PFS 5 -Yr PFS Cumulative Probability 1. 0 5 -Yr OS 96% 1. 0 80% 76% 0. 8 0. 6 46% 0. 4 0. 2 0 OS 0 FLIPI-2 Score No. (%) 0 (low risk) 42 (18) 1 -2 (intermediate risk) 143 (62) 3 -5 (high risk) 46 (20) 6 12 18 24 30 Mos Federico. JCO. 2009; 27: 4555. 29% 42 48 54 0. 6 0. 4 0. 2 Log‐rank P =. 0005 36 59% 60 0 Log‐rank P =. 0003 0 6 12 18 24 30 36 Mos 42 48 54 60 Slide credit: clinicaloptions. com

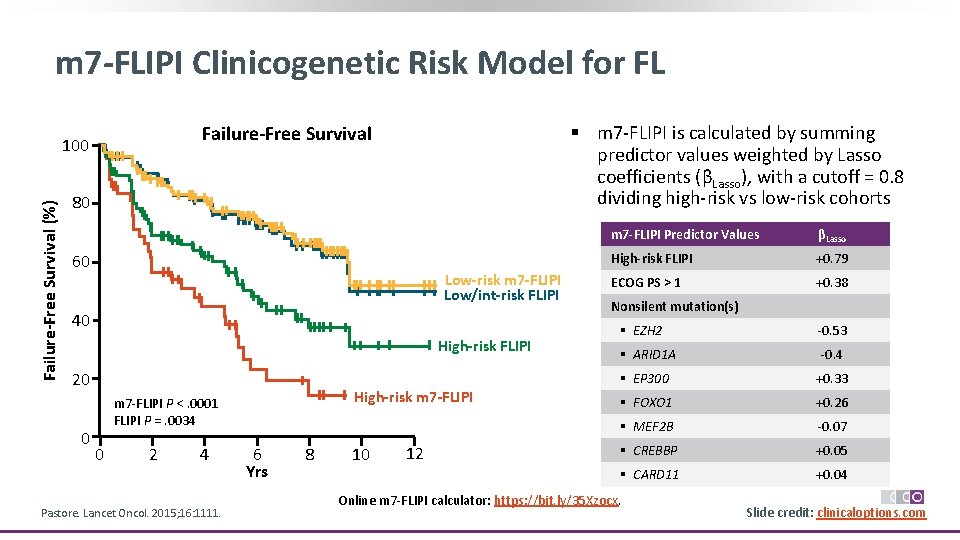
m 7 -FLIPI Clinicogenetic Risk Model for FL Failure-Free Survival (%) § m 7‐FLIPI is calculated by summing predictor values weighted by Lasso coefficients (βLasso), with a cutoff = 0. 8 dividing high‐risk vs low‐risk cohorts Failure-Free Survival 100 80 60 Low-risk m 7 -FLIPI Low/int-risk FLIPI 40 High-risk FLIPI 20 0 High-risk m 7 -FLIPI m 7‐FLIPI P <. 0001 FLIPI P =. 0034 0 2 4 Pastore. Lancet Oncol. 2015; 16: 1111. 6 Yrs 8 10 12 m 7 -FLIPI Predictor Values βLasso High-risk FLIPI +0. 79 ECOG PS > 1 +0. 38 Nonsilent mutation(s) § EZH 2 ‐ 0. 53 § ARID 1 A ‐ 0. 4 § EP 300 +0. 33 § FOXO 1 +0. 26 § MEF 2 B ‐ 0. 07 § CREBBP +0. 05 § CARD 11 +0. 04 Online m 7‐FLIPI calculator: https: //bit. ly/35 Xzocx. Slide credit: clinicaloptions. com

Treatment Considerations for Newly Diagnosed FL § Disease stage § CR and/or prolonged PFS § Tumor grade ‒ Prioritizes longer remissions over Qo. L § Tumor burden ‒ Usually requires more aggressive treatment that is more toxic in the short term § Symptoms § Patient age and fitness § Patient goals and priorities § Maximize Qo. L and/or reduce risk for AEs ‒ Prioritizes Qo. L over longer remissions ‒ Usually involves gentler treatment with fewer toxicities at the expense of efficacy Blinman. Ann Oncol. 2012; 23: 1104. Dreyling. Ann Oncol. 2016; 27(suppl 5): v 83. Meropol. Cancer. 2008; 113: 3459. Slide credit: clinicaloptions. com

GELF Criteria § A person has high tumor burden if they have ≥ 1 of the following: ‒ Any mass ≥ 7 cm in diameter ‒ Involvement of ≥ 3 LNs, each ≥ 3 cm in diameter ‒ Presence of B symptoms ‒ Splenomegaly ‒ Compression syndrome (ureteral, orbital, GI) ‒ Ascites or pleural effusion ‒ Cytopenias (WBC < 1 x 109/L or PLTs < 100 x 109/L) ‒ Leukemia (> 5. 0 x 109/L circulating malignant cells) ‒ (Elevated LDH or β 2‐microglobulin) Solal‐Céligny. JCO. 1998; 16: 2332. Brice. JCO. 1997; 15: 1110. Dreyling. Ann Oncol. 2016; 27(suppl 5): v 83. Slide credit: clinicaloptions. com

Key Studies Informing How to Manage Low Tumor Burden, Asymptomatic Follicular Lymphoma § Ardeshna Study: In asymptomatic, advanced stage FL, rituximab therapy improves PFS but not OS compared with watchful waiting[1] § RESORT: In patients with low tumor burden FL, maintenance rituximab improves PFS but not OS compared with no maintenance[2] § SAKK 35/98: In patients with low tumor burden FL, short‐duration maintenance rituximab improves EFS compared with no maintenance[3] 1. Ardeshna. Lancet Oncol. 2014; 15: 424. 2. Kahl. JCO. 2014; 32: 3096. 3. Martinelli. JCO. 2010; 28: 4480. Slide credit: clinicaloptions. com

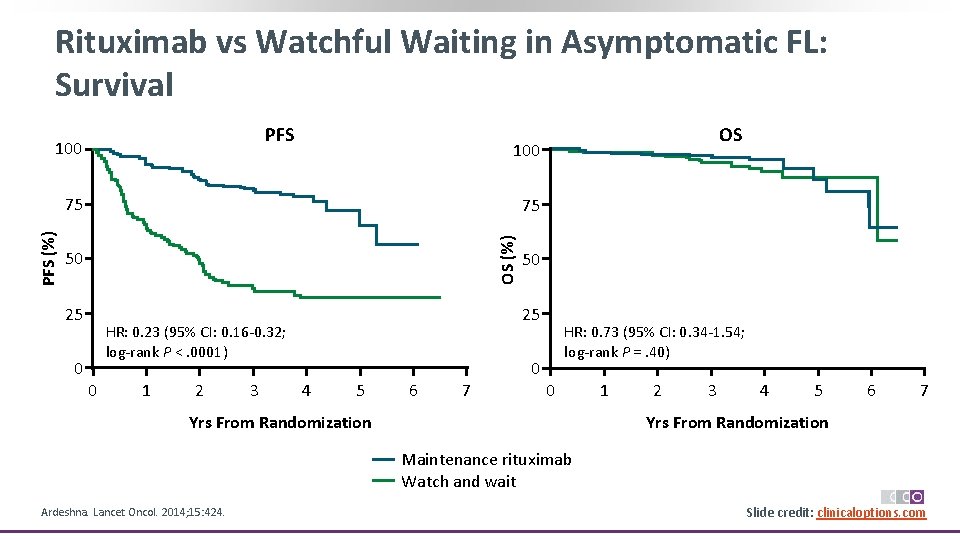
Rituximab vs Watchful Waiting in Asymptomatic FL: Is Treatment Needed? § Randomized phase III study Stratified by age, grade, stage, and institution Patients with asymptomatic stage II, IV FL with low tumor burden (N = 463) Mo 3 Mo 7 CT scan* Mo 13 CT scan if clinical CR* Rituximab 375 mg/m 2 wkly for 4 wks (n = 192) Rituximab 375 mg/m 2 every 2 mos for 2 yrs Rituximab 375 mg/m 2 wkly for 4 wks (n = 84) Regular clinic visits Mo 25 CT scan* Continued follow-up Watchful waiting with regular clinic visits (n = 187) *If CT shows CR, bone marrow biopsied for restaging. Ardeshna. Lancet Oncol. 2014; 15: 424. Slide credit: clinicaloptions. com

Rituximab vs Watchful Waiting in Asymptomatic FL: Survival PFS 100 75 75 OS (%) PFS (%) OS 50 25 25 HR: 0. 23 (95% CI: 0. 16‐ 0. 32; log‐rank P <. 0001) 0 0 1 2 3 50 HR: 0. 73 (95% CI: 0. 34‐ 1. 54; log‐rank P =. 40) 0 4 5 6 7 0 Yrs From Randomization 1 2 3 4 5 6 7 Yrs From Randomization Maintenance rituximab Watch and wait Ardeshna. Lancet Oncol. 2014; 15: 424. Slide credit: clinicaloptions. com

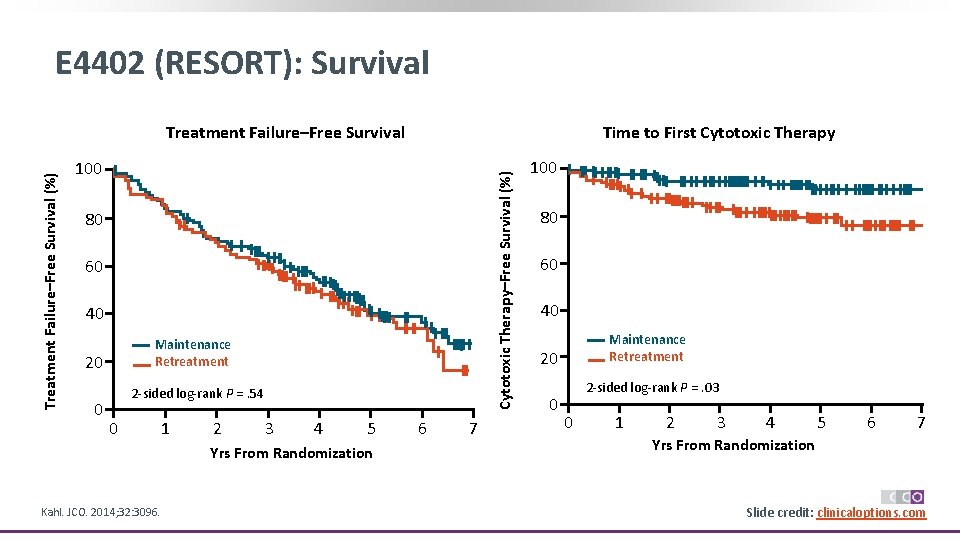
E 4402 (RESORT) Trial: Maintenance Rituximab vs Retreatment in Low Tumor Burden FL § Randomized phase III trial Stratified by age (< 60 vs ≥ 60 yrs) and time from diagnosis (< 1 vs ≥ 1 yr) Maintenance Patients with FL and low tumor burden who received frontline rituximab 375 mg/m 2/wk for 4 wks (N = 408) Patients with CR or PR (N = 299) § Primary endpoint: TTF Rituximab 375 mg/m 2 Q 13 W (n = 148*) Retreatment at Progression Rituximab 375 mg/m 2 wkly x 4 (n = 151†) Continue until rituximab treatment failure Median follow-up: 4. 5 yrs *n = 143 assessed. †n = 146 assessed. § Secondary endpoints: time to first cytotoxic chemotherapy, safety/toxicity, Qo. L Kahl. JCO. 2014; 32: 3096. Slide credit: clinicaloptions. com

E 4402 (RESORT): Survival Time to First Cytotoxic Therapy 100 Cytotoxic Therapy–Free Survival (%) Treatment Failure–Free Survival 80 60 40 Maintenance Retreatment 20 0 2‐sided log‐rank P =. 54 0 1 2 3 4 5 Yrs From Randomization Kahl. JCO. 2014; 32: 3096. 6 7 100 80 60 40 Maintenance Retreatment 20 0 2‐sided log‐rank P =. 03 0 1 2 3 4 5 6 7 Yrs From Randomization Slide credit: clinicaloptions. com

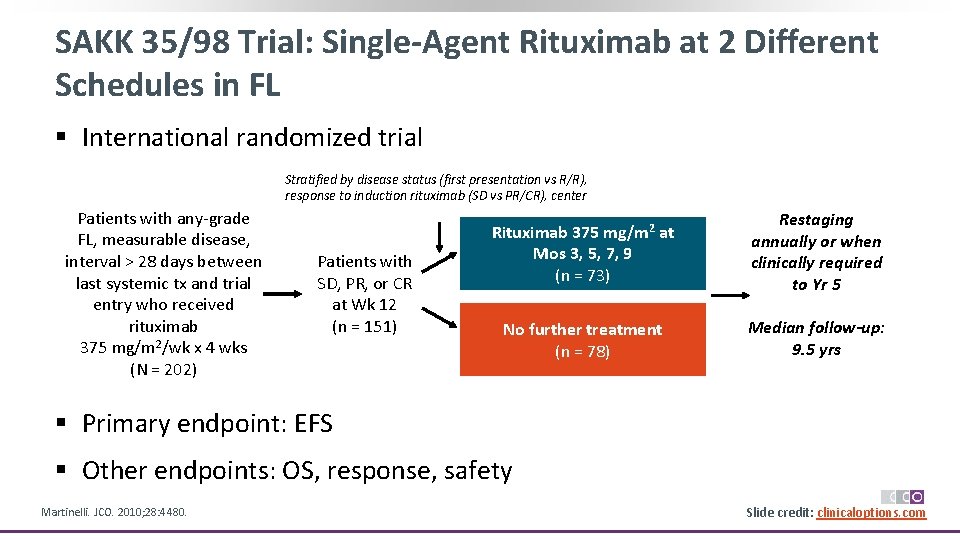
SAKK 35/98 Trial: Single-Agent Rituximab at 2 Different Schedules in FL § International randomized trial Stratified by disease status (first presentation vs R/R), response to induction rituximab (SD vs PR/CR), center Patients with any‐grade FL, measurable disease, interval > 28 days between last systemic tx and trial entry who received rituximab 375 mg/m 2/wk x 4 wks (N = 202) Patients with SD, PR, or CR at Wk 12 (n = 151) Rituximab 375 mg/m 2 at Mos 3, 5, 7, 9 (n = 73) Restaging annually or when clinically required to Yr 5 No further treatment (n = 78) Median follow-up: 9. 5 yrs § Primary endpoint: EFS § Other endpoints: OS, response, safety Martinelli. JCO. 2010; 28: 4480. Slide credit: clinicaloptions. com

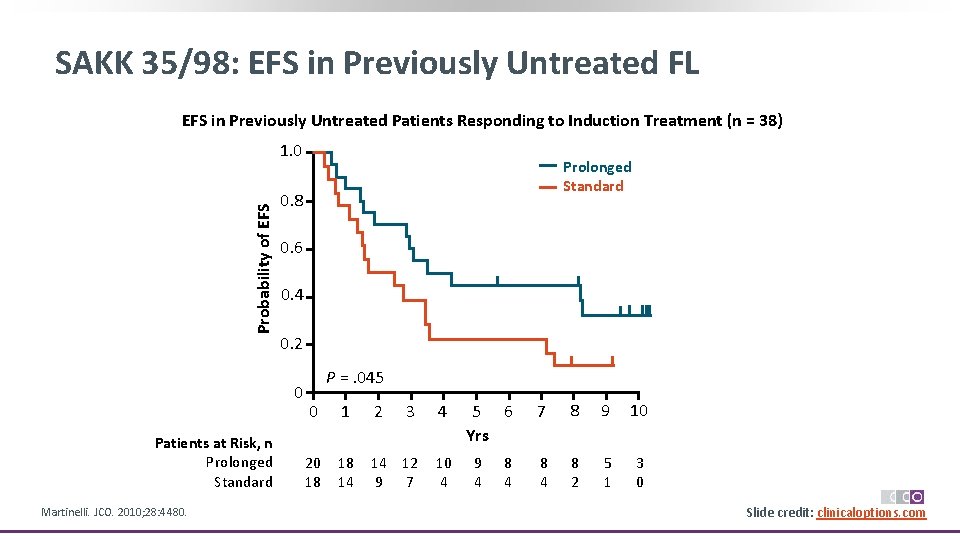
SAKK 35/98: EFS in Previously Untreated FL EFS in Previously Untreated Patients Responding to Induction Treatment (n = 38) Probability of EFS 1. 0 0. 8 0. 6 0. 4 0. 2 0 Patients at Risk, n Prolonged Standard Martinelli. JCO. 2010; 28: 4480. Prolonged Standard P =. 045 0 1 2 3 4 20 18 18 14 14 12 9 7 10 4 5 6 Yrs 9 4 8 4 7 8 9 10 8 4 8 2 5 1 3 0 Slide credit: clinicaloptions. com

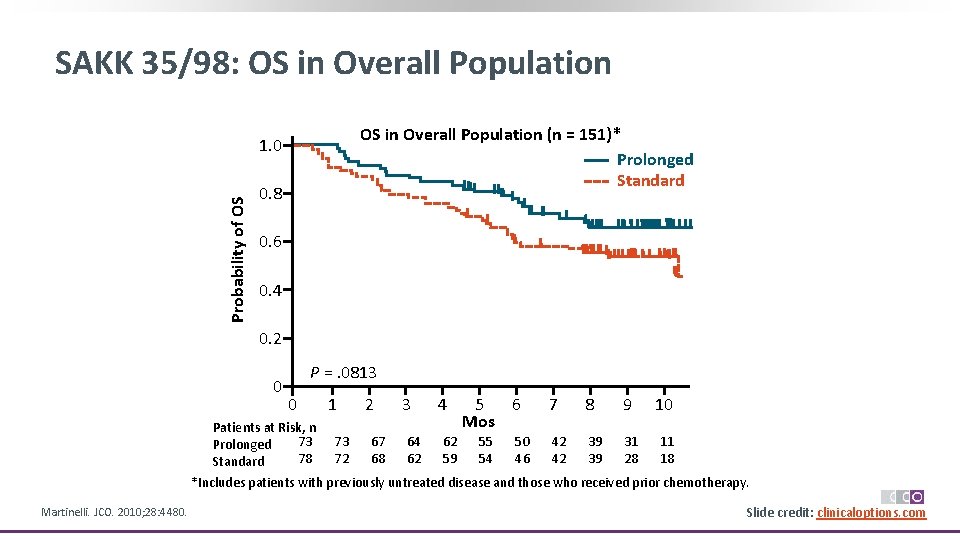
SAKK 35/98: OS in Overall Population (n = 151)* Prolonged Standard Probability of OS 1. 0 0. 8 0. 6 0. 4 0. 2 0 P =. 0813 0 1 2 3 4 5 6 Mos 7 8 9 10 Patients at Risk, n 73 73 67 64 62 55 50 42 39 31 11 Prolonged 78 72 68 62 59 54 46 42 39 28 18 Standard *Includes patients with previously untreated disease and those who received prior chemotherapy. Martinelli. JCO. 2010; 28: 4480. Slide credit: clinicaloptions. com

Trials of Chemoimmunotherapy for Newly Diagnosed FL § Comparison of chemotherapy backbone in rituximab‐based chemoimmunotherapy ‒ FOLL 05: R‐CVP vs R‐CHOP vs R‐FM ‒ Sti. L NHL 1 -2003: BR vs R‐CHOP ‒ BRIGHT: BR vs R‐CHOP or R‐CVP § Obinutuzumab‐based vs rituximab‐based chemoimmunotherapy ‒ GALLIUM

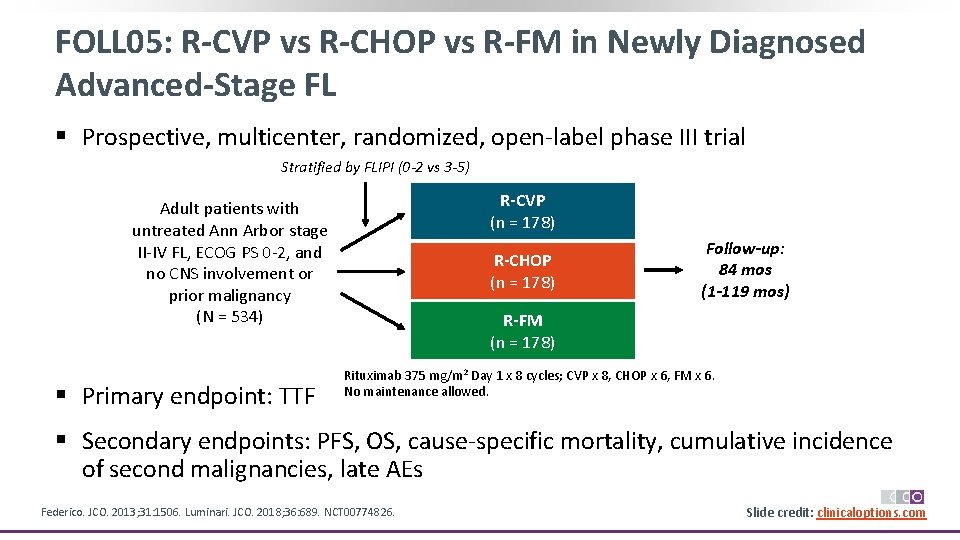
FOLL 05: R-CVP vs R-CHOP vs R-FM in Newly Diagnosed Advanced-Stage FL § Prospective, multicenter, randomized, open‐label phase III trial Stratified by FLIPI (0 -2 vs 3 -5) R-CVP (n = 178) Adult patients with untreated Ann Arbor stage II‐IV FL, ECOG PS 0‐ 2, and no CNS involvement or prior malignancy (N = 534) § Primary endpoint: TTF R-CHOP (n = 178) Follow-up: 84 mos (1 -119 mos) R-FM (n = 178) Rituximab 375 mg/m 2 Day 1 x 8 cycles; CVP x 8, CHOP x 6, FM x 6. No maintenance allowed. § Secondary endpoints: PFS, OS, cause‐specific mortality, cumulative incidence of second malignancies, late AEs Federico. JCO. 2013; 31: 1506. Luminari. JCO. 2018; 36: 689. NCT 00774826. Slide credit: clinicaloptions. com

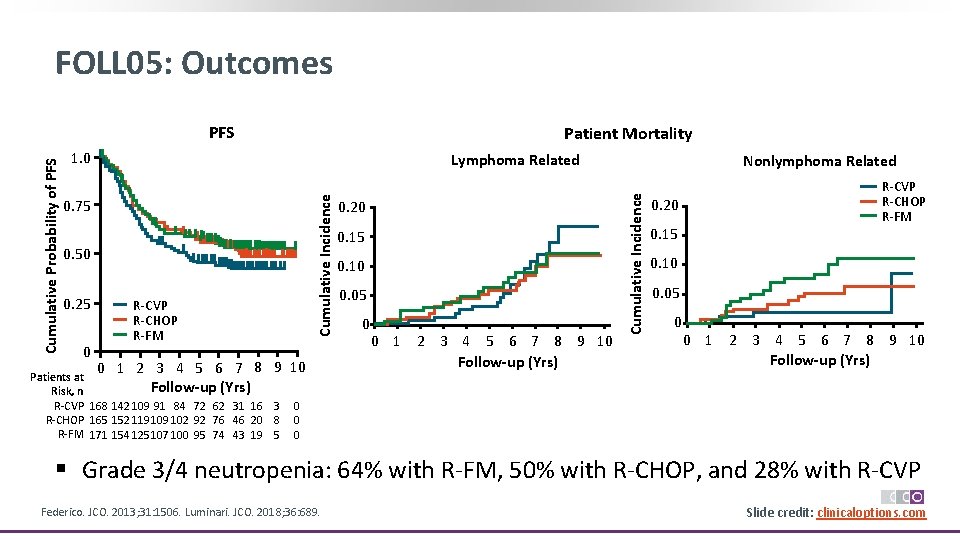
FOLL 05: Outcomes Patient Mortality 1. 0 Lymphoma Related 0. 75 0. 50 0. 25 0 R‐CVP R‐CHOP R‐FM 0 1 2 3 4 5 6 7 8 9 10 Patients at 10 Follow-up (Yrs) Risk, n R‐CVP 168 142 109 91 84 72 62 31 16 3 R‐CHOP 165 152 119 102 92 76 46 20 8 R‐FM 171 154 125 107 100 95 74 43 19 5 0. 20 0. 15 0. 10 0. 05 0 0 1 2 3 4 5 6 7 8 9 10 Follow-up (Yrs) Nonlymphoma Related Cumulative Incidence Cumulative Probability of PFS R‐CVP R‐CHOP R‐FM 0. 20 0. 15 0. 10 0. 05 0 0 1 2 3 4 5 6 7 8 9 10 Follow-up (Yrs) 0 0 0 § Grade 3/4 neutropenia: 64% with R‐FM, 50% with R‐CHOP, and 28% with R‐CVP Federico. JCO. 2013; 31: 1506. Luminari. JCO. 2018; 36: 689. Slide credit: clinicaloptions. com
![Sti L NHL 1 2003 BR vs RCHOP in Newly Diagnosed FL PFS1 Median Sti. L NHL 1 -2003: BR vs R-CHOP in Newly Diagnosed FL PFS[1] Median](https://slidetodoc.com/presentation_image_h/0c8e0d12fcfbde4a8ffd2b11d8b94ff1/image-29.jpg)
Sti. L NHL 1 -2003: BR vs R-CHOP in Newly Diagnosed FL PFS[1] Median PFS, Mos (IQR) BR NR (22. 1‐NR) R‐CHOP 40. 9 (15. 2‐NR) § Randomized, open‐label phase III noninferiority trial ‒ Primary endpoint: noninferiority of BR vs R‐CHOP for PFS (decrease < 10% at 3 yrs) Stratified by histological subtype BR (n = 274*) 0. 8 0. 6 0. 4 0. 2 0. 0 HR: 0. 61 (95% CI: 0. 42‐ 0. 87; P =. 0072) Median f/u: 45 mos 0 12 24 R-CHOP (n = 275†) *n = 261 assessed. †n = 253 assessed. § No OS difference between arms § Toxicity less with BR (SAEs: 19% vs 29% with R‐CHOP) 1. Rummel. Lancet. 2013; 381: 1203. 2. Rummel. ASCO 2017. Abstr 7501. 36 48 60 Mos TTNT[2] 1 Probability of TTNT Treatment‐naive patients with MCL or indolent CD 20‐positive lymphoma, including FL (N = 549) Probability of PFS 1. 0 0. 75 72 84 96 Median TTNT, Mos BR NR R‐CHOP 56. 0 0. 5 0. 25 0 HR: 0. 55 (95% CI: 0. 41‐ 0. 73; P <. 0001) Median f/u: 117 mos 0 24 48 96 120 144 168 Mos Slide credit: clinicaloptions. com 72

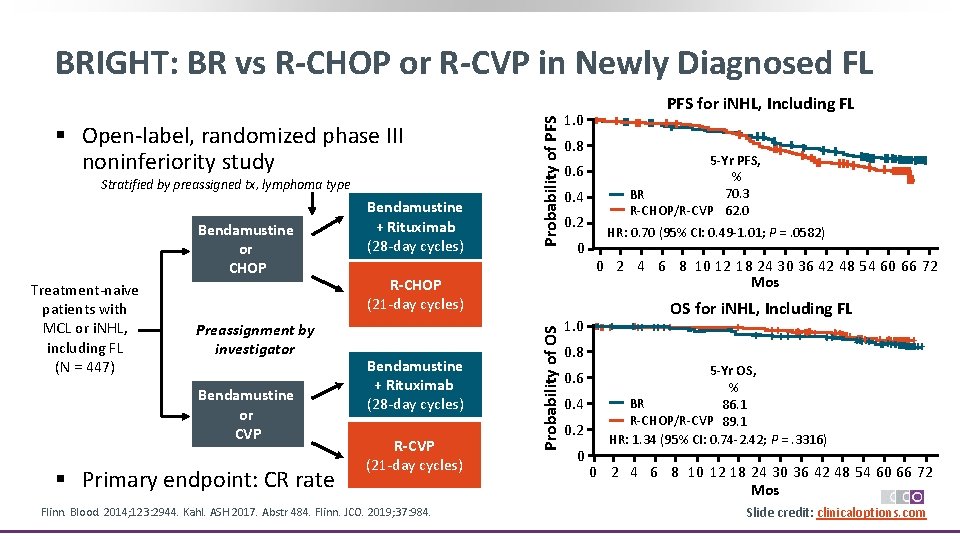
§ Open‐label, randomized phase III noninferiority study Stratified by preassigned tx, lymphoma type Treatment‐naive patients with MCL or i. NHL, including FL (N = 447) Preassignment by investigator Bendamustine or CVP § Primary endpoint: CR rate 1. 0 0. 8 0. 6 0. 4 0. 2 0 R-CHOP (21‐day cycles) Bendamustine + Rituximab (28‐day cycles) R-CVP (21‐day cycles) Flinn. Blood. 2014; 123: 2944. Kahl. ASH 2017. Abstr 484. Flinn. JCO. 2019; 37: 984. PFS for i. NHL, Including FL 5 -Yr PFS, % 70. 3 BR R‐CHOP/R‐CVP 62. 0 HR: 0. 70 (95% CI: 0. 49‐ 1. 01; P =. 0582) 0 2 4 6 8 10 12 18 24 30 36 42 48 54 60 66 72 Mos OS for i. NHL, Including FL Probability of OS Bendamustine or CHOP Bendamustine + Rituximab (28‐day cycles) Probability of PFS BRIGHT: BR vs R-CHOP or R-CVP in Newly Diagnosed FL 1. 0 +++ + + ++++ 0. 8 0. 6 0. 4 0. 2 0 + + ++ ++++++ +++++++++++ 5 -Yr OS, % BR 86. 1 R‐CHOP/R‐CVP 89. 1 HR: 1. 34 (95% CI: 0. 74‐ 2. 42; P =. 3316) 0 2 4 6 8 10 12 18 24 30 36 42 48 54 60 66 72 Mos Slide credit: clinicaloptions. com

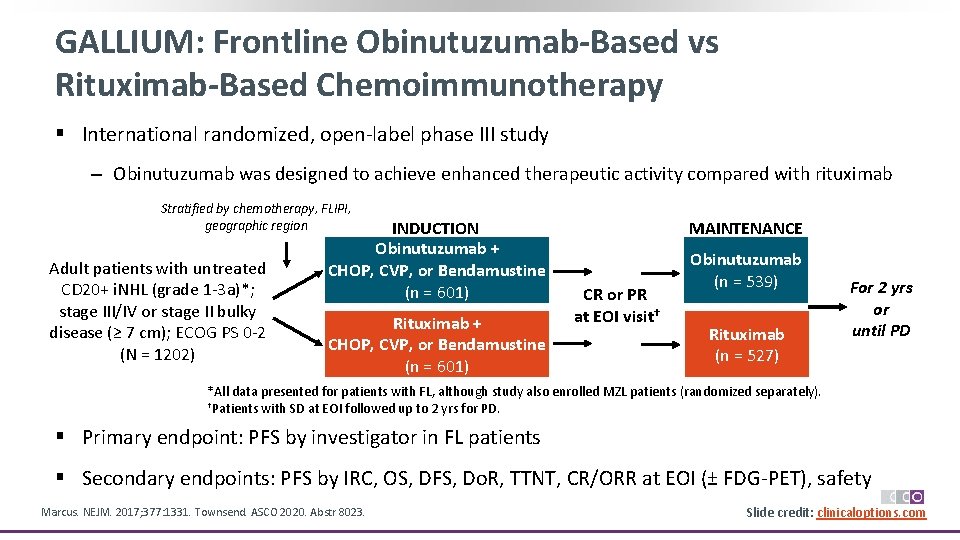
GALLIUM: Frontline Obinutuzumab-Based vs Rituximab-Based Chemoimmunotherapy § International randomized, open‐label phase III study ‒ Obinutuzumab was designed to achieve enhanced therapeutic activity compared with rituximab Stratified by chemotherapy, FLIPI, geographic region Adult patients with untreated CD 20+ i. NHL (grade 1‐ 3 a)*; stage III/IV or stage II bulky disease (≥ 7 cm); ECOG PS 0‐ 2 (N = 1202) INDUCTION Obinutuzumab + CHOP, CVP, or Bendamustine (n = 601) Rituximab + CHOP, CVP, or Bendamustine (n = 601) MAINTENANCE CR or PR at EOI visit† Obinutuzumab (n = 539) Rituximab (n = 527) For 2 yrs or until PD *All data presented for patients with FL, although study also enrolled MZL patients (randomized separately). †Patients with SD at EOI followed up to 2 yrs for PD. § Primary endpoint: PFS by investigator in FL patients § Secondary endpoints: PFS by IRC, OS, DFS, Do. R, TTNT, CR/ORR at EOI (± FDG‐PET), safety Marcus. NEJM. 2017; 377: 1331. Townsend. ASCO 2020. Abstr 8023. Slide credit: clinicaloptions. com

GALLIUM: Updated Investigator-Assessed PFS Probability of PFS 1. 0 0. 8 5 -Yr PFS, % 0. 6 0. 4 0. 2 0 Patients at Risk, n Obinutuzumab + CT Rituximab + CT Obinutuzumab + CT 70. 5 Rituximab + CT 63. 2 HR: 0. 76 (95% CI: 0. 62‐ 0. 92); P =. 0043 + Censored 0 Median follow‐up: 76. 5 mos Data cutoff: October 31, 2019 6 12 18 24 30 36 42 48 56 60 66 72 78 84 90 96 Mos 601 574 539 512 491 467 446 430 406 368 334 269 182 98 53 4 601 563 512 471 447 429 404 373 349 328 304 247 176 88 52 3 § Numerical decrease in POD 24 with obinutuzumab + CT vs rituximab + CT (9. 2% vs 16. 3%) § No significant difference in 5‐yr OS between arms (90. 2% vs 89. 4%, respectively; HR: 0. 87; 95% CI: 0. 62‐ 1. 22; P =. 41) Townsend. ASCO 2020. Abstr 8023. Slide credit: clinicaloptions. com

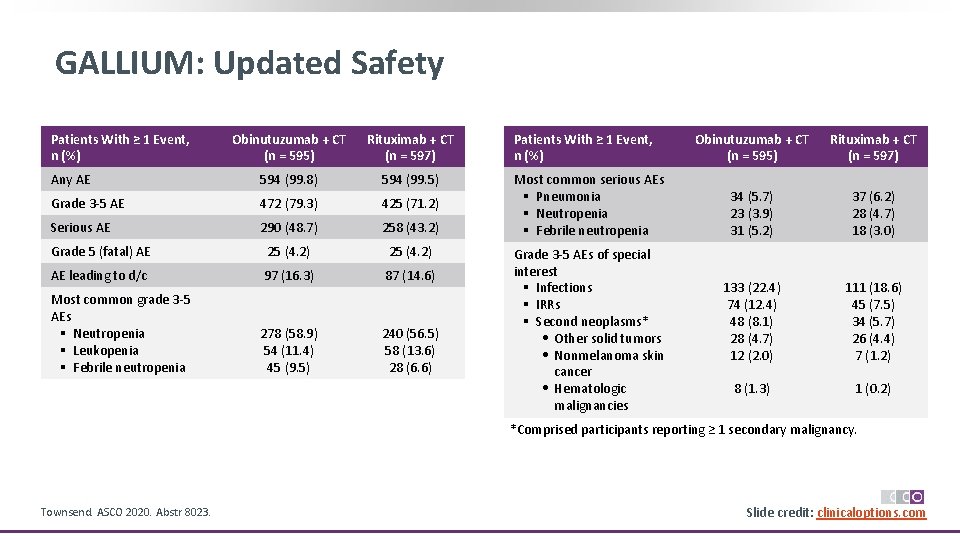
GALLIUM: Updated Safety Patients With ≥ 1 Event, n (%) Obinutuzumab + CT (n = 595) Rituximab + CT (n = 597) Any AE 594 (99. 8) 594 (99. 5) Grade 3‐ 5 AE 472 (79. 3) 425 (71. 2) Serious AE 290 (48. 7) 258 (43. 2) Grade 5 (fatal) AE 25 (4. 2) AE leading to d/c 97 (16. 3) 87 (14. 6) Most common grade 3‐ 5 AEs § Neutropenia § Leukopenia § Febrile neutropenia 278 (58. 9) 54 (11. 4) 45 (9. 5) 240 (56. 5) 58 (13. 6) 28 (6. 6) Patients With ≥ 1 Event, n (%) Most common serious AEs § Pneumonia § Neutropenia § Febrile neutropenia Grade 3‐ 5 AEs of special interest § Infections § IRRs § Second neoplasms* • Other solid tumors • Nonmelanoma skin cancer • Hematologic malignancies Obinutuzumab + CT (n = 595) Rituximab + CT (n = 597) 34 (5. 7) 23 (3. 9) 31 (5. 2) 37 (6. 2) 28 (4. 7) 18 (3. 0) 133 (22. 4) 74 (12. 4) 48 (8. 1) 28 (4. 7) 12 (2. 0) 111 (18. 6) 45 (7. 5) 34 (5. 7) 26 (4. 4) 7 (1. 2) 8 (1. 3) 1 (0. 2) *Comprised participants reporting ≥ 1 secondary malignancy. Townsend. ASCO 2020. Abstr 8023. Slide credit: clinicaloptions. com

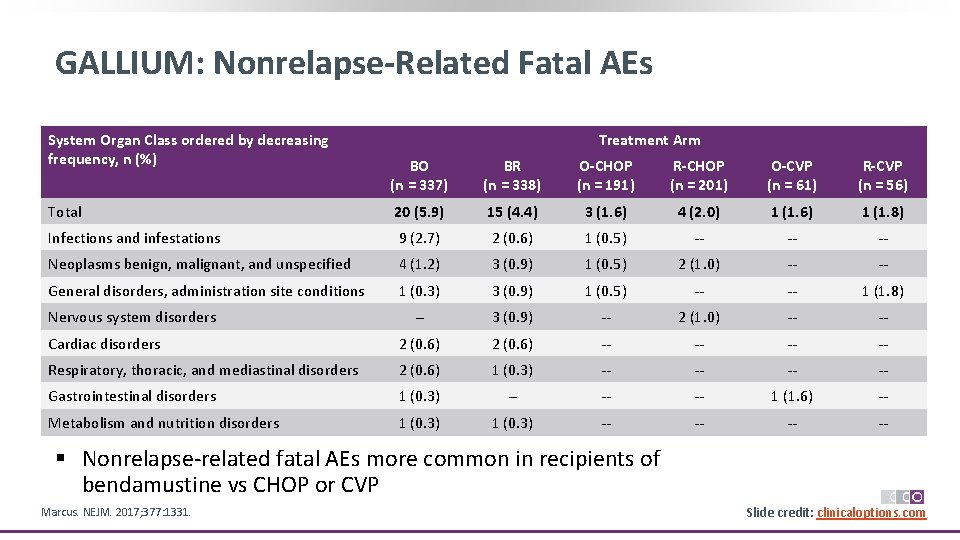
GALLIUM: Nonrelapse-Related Fatal AEs System Organ Class ordered by decreasing frequency, n (%) Treatment Arm BO (n = 337) BR (n = 338) O-CHOP (n = 191) R-CHOP (n = 201) O-CVP (n = 61) R-CVP (n = 56) Total 20 (5. 9) 15 (4. 4) 3 (1. 6) 4 (2. 0) 1 (1. 6) 1 (1. 8) Infections and infestations 9 (2. 7) 2 (0. 6) 1 (0. 5) ‐‐ ‐‐ ‐‐ Neoplasms benign, malignant, and unspecified 4 (1. 2) 3 (0. 9) 1 (0. 5) 2 (1. 0) ‐‐ ‐‐ General disorders, administration site conditions 1 (0. 3) 3 (0. 9) 1 (0. 5) ‐‐ ‐‐ 1 (1. 8) ‐‐ 3 (0. 9) ‐‐ 2 (1. 0) ‐‐ ‐‐ Cardiac disorders 2 (0. 6) ‐‐ ‐‐ Respiratory, thoracic, and mediastinal disorders 2 (0. 6) 1 (0. 3) ‐‐ ‐‐ Gastrointestinal disorders 1 (0. 3) ‐‐ ‐‐ ‐‐ 1 (1. 6) ‐‐ Metabolism and nutrition disorders 1 (0. 3) ‐‐ ‐‐ Nervous system disorders § Nonrelapse‐related fatal AEs more common in recipients of bendamustine vs CHOP or CVP Marcus. NEJM. 2017; 377: 1331. Slide credit: clinicaloptions. com

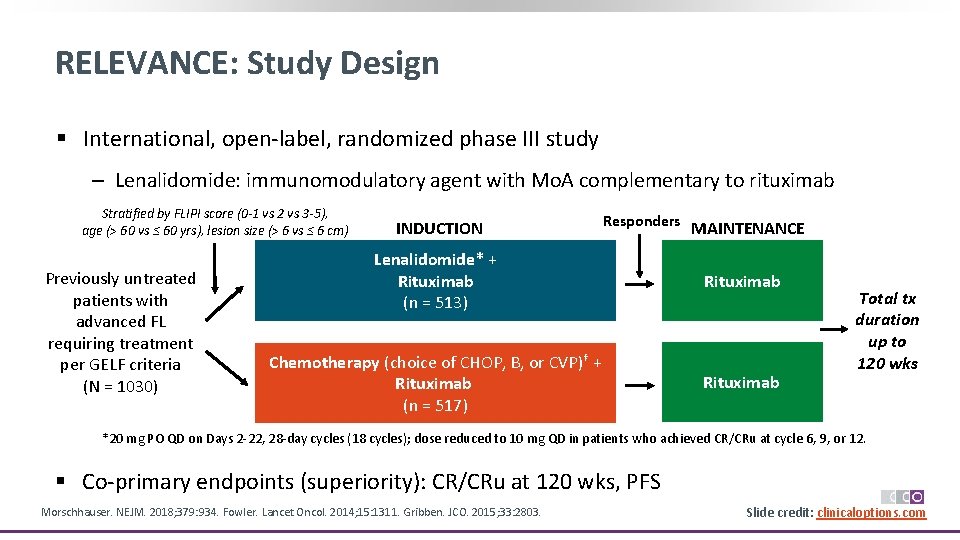
RELEVANCE: Study Design § International, open‐label, randomized phase III study ‒ Lenalidomide: immunomodulatory agent with Mo. A complementary to rituximab Stratified by FLIPI score (0 -1 vs 2 vs 3 -5), age (> 60 vs ≤ 60 yrs), lesion size (> 6 vs ≤ 6 cm) Previously untreated patients with advanced FL requiring treatment per GELF criteria (N = 1030) INDUCTION Responders MAINTENANCE Lenalidomide* + Rituximab (n = 513) Rituximab Chemotherapy (choice of CHOP, B, or CVP)† + Rituximab (n = 517) Rituximab Total tx duration up to 120 wks *20 mg PO QD on Days 2‐ 22, 28‐day cycles (18 cycles); dose reduced to 10 mg QD in patients who achieved CR/CRu at cycle 6, 9, or 12. § Co‐primary endpoints (superiority): CR/CRu at 120 wks, PFS Morschhauser. NEJM. 2018; 379: 934. Fowler. Lancet Oncol. 2014; 15: 1311. Gribben. JCO. 2015; 33: 2803. Slide credit: clinicaloptions. com

RELEVANCE: PFS by IRC PFS Probability (IRC) 1. 0 Co-Primary Endpoint: Interim PFS (~ 50% Events) R 2 R-CT 0. 8 Patients, n 513 517 0. 6 3‐yr PFS, % (95% CI) 77 (72‐ 80) 78 (74‐ 82) § Interim PFS at median follow‐up of 37. 9 mos was similar in both arms 0. 4 0. 2 0 HR: 1. 10 (95% CI: 0. 85‐ 1. 43; P =. 48) 0 6 12 18 24 30 36 42 48 54 60 66 Mos From Randomization Patients at Risk, n R 2 513 435 409 393 364 282 174 107 49 13 0 R-CT 517 474 446 417 387 287 175 109 51 14 1 Morschhauser. NEJM. 2018; 379: 934. 0 Slide credit: clinicaloptions. com

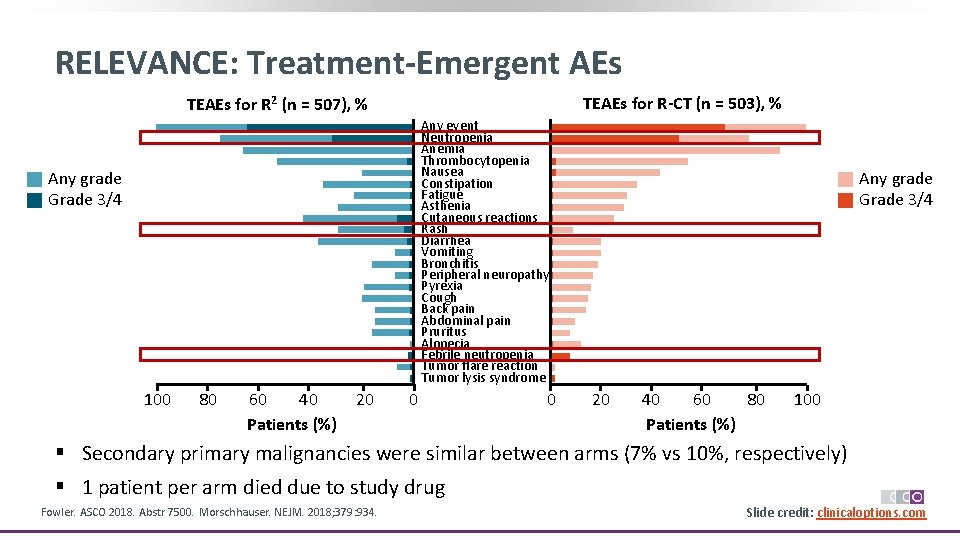
RELEVANCE: Treatment-Emergent AEs TEAEs for R-CT (n = 503), % TEAEs for R 2 (n = 507), % Any event Neutropenia Anemia Thrombocytopenia Nausea Constipation Fatigue Asthenia Cutaneous reactions Rash Diarrhea Vomiting Bronchitis Peripheral neuropathy Pyrexia Cough Back pain Abdominal pain Pruritus Alopecia Febrile neutropenia Tumor flare reaction Tumor lysis syndrome Any grade Grade 3/4 100 80 60 40 Patients (%) 20 0 0 Any grade Grade 3/4 20 40 60 80 Patients (%) 100 § Secondary primary malignancies were similar between arms (7% vs 10%, respectively) § 1 patient per arm died due to study drug Fowler. ASCO 2018. Abstr 7500. Morschhauser. NEJM. 2018; 379: 934. Slide credit: clinicaloptions. com

Alternate Forms of Rituximab

What Is a Biosimilar? § Biologic drug is highly similar to licensed (ie, reference or originator) biologic product notwithstanding minor differences in clinically inactive components § No clinically meaningful differences between biologic product and reference product in terms of safety, purity, and potency § Approval of biosimilars is based on the totality of the data supporting biosimilarity Lyman. NEJM. 2018; 378: 2036. Stepwise Development: Totality of the Evidence Clinical If uncertainty remains PK/PD & Immunogenicity Preclinical Analytical Slide credit: clinicaloptions. com

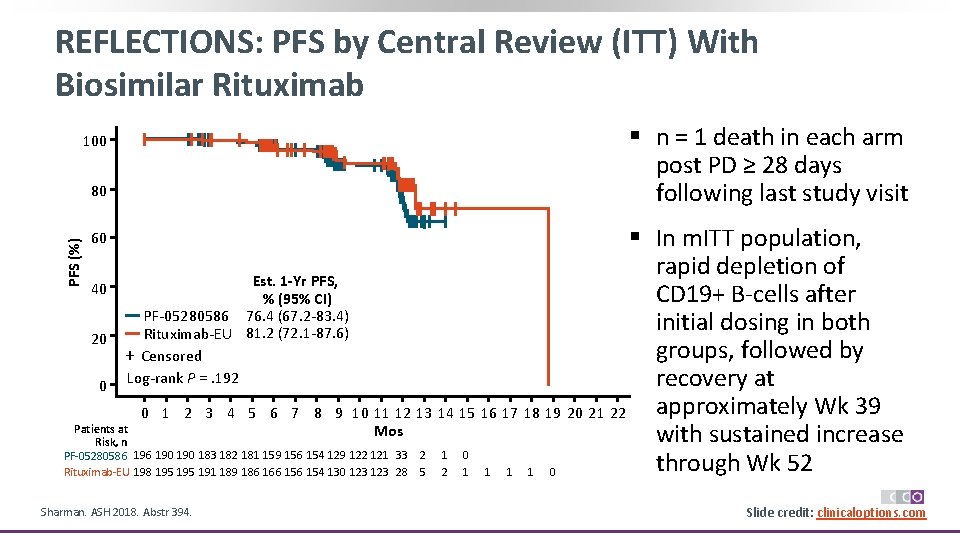
REFLECTIONS: PFS by Central Review (ITT) With Biosimilar Rituximab 100 80 § n = 1 death in each arm post PD ≥ 28 days following last study visit PFS (%) § In m. ITT population, rapid depletion of Est. 1 -Yr PFS, 40 CD 19+ B‐cells after % (95% CI) PF‐ 05280586 76. 4 (67. 2‐ 83. 4) initial dosing in both Rituximab‐EU 81. 2 (72. 1‐ 87. 6) 20 groups, followed by + Censored recovery at 0 Log‐rank P =. 192 approximately Wk 39 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Patients at Mos with sustained increase Risk, n PF‐ 05280586 190 190 183 182 181 159 156 154 129 122 121 33 2 1 0 through Wk 52 Rituximab‐EU 198 195 191 189 186 166 154 130 123 28 5 2 1 1 0 60 Sharman. ASH 2018. Abstr 394. Slide credit: clinicaloptions. com

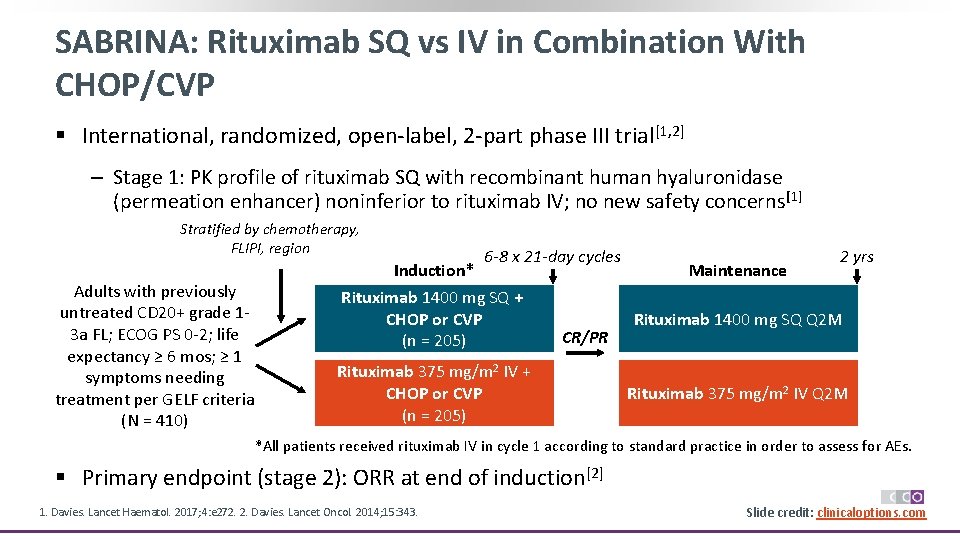
SABRINA: Rituximab SQ vs IV in Combination With CHOP/CVP § International, randomized, open‐label, 2‐part phase III trial[1, 2] ‒ Stage 1: PK profile of rituximab SQ with recombinant human hyaluronidase (permeation enhancer) noninferior to rituximab IV; no new safety concerns [1] Stratified by chemotherapy, FLIPI, region Adults with previously untreated CD 20+ grade 1‐ 3 a FL; ECOG PS 0‐ 2; life expectancy ≥ 6 mos; ≥ 1 symptoms needing treatment per GELF criteria (N = 410) Induction* 6 -8 x 21 -day cycles Rituximab 1400 mg SQ + CHOP or CVP (n = 205) CR/PR Rituximab 375 mg/m 2 IV + CHOP or CVP (n = 205) Maintenance 2 yrs Rituximab 1400 mg SQ Q 2 M Rituximab 375 mg/m 2 IV Q 2 M *All patients received rituximab IV in cycle 1 according to standard practice in order to assess for AEs. § Primary endpoint (stage 2): ORR at end of induction[2] 1. Davies. Lancet Haematol. 2017; 4: e 272. 2. Davies. Lancet Oncol. 2014; 15: 343. Slide credit: clinicaloptions. com
![SABRINA Comparable Efficacy Safety of Rituximab SQ vs IV CHOPCVP Response n 1 SABRINA: Comparable Efficacy, Safety of Rituximab SQ vs IV + CHOP/CVP Response, n (%)[1]](https://slidetodoc.com/presentation_image_h/0c8e0d12fcfbde4a8ffd2b11d8b94ff1/image-42.jpg)
SABRINA: Comparable Efficacy, Safety of Rituximab SQ vs IV + CHOP/CVP Response, n (%)[1] Rituximab SQ Rituximab IV Induction n = 205 § ORR 173 (84. 4) 174 (84. 9) § CR/CRu 66 (32. 2) Maintenance n = 172 n = 178 § ORR 134 (77. 9) 139 (78. 1) § CR/CRu 87 (50. 6) 100 (56. 2) Rituximab SQ (n = 197) Rituximab IV (n = 210*) AE 189 (96) 199 (95) Serious AE 73 (37) 72 (34) Grade ≥ 3 AE 111 (56) 116 (55) Administration‐related reaction 95 (48) 73 (35) Safety Outcome, n (%)[1] *Includes safety data from 6 patients assigned to rituximab SQ arm who withdrew after cycle 1, when all patients received rituximab IV. § Response rates did not substantially differ by type of chemotherapy or body surface area [1] § After median f/u of 37 mos, no significant differences observed between arms for PFS, EFS, OS [1] § In 2017, FDA approved rituximab SQ with hyaluronidase for FL in combination with first‐line chemotherapy, and after achieving CR/PR, as single‐agent maintenance therapy; as monotherapy for R/R FL; and as single‐agent therapy for nonprogressing FL after first‐line CVP [2] 1. Davies. Lancet Haematol. 2017; 4: e 272. 2. Rituximab and hyaluronidase SQ PI. Slide credit: clinicaloptions. com

Frontline FL: Conclusions § Outcomes for patients with untreated, advanced FL have improved substantially in the era of rituximab‐based strategies ‒ Median survival is ~ 20 yrs § Advances in the first‐line treatment of FL continue to prolong PFS ‒ Obinutuzumab‐based CIT with maintenance decreased risk of PD or death by 24% vs rituximab‐based therapy, with increased risk of grade 3/4 cytopenias and IRRs § Lenalidomide + rituximab is a reasonable option for patients with FL wishing to avoid CT § Alternate forms of rituximab are now available, including biosimilars and an SQ formulation Slide credit: clinicaloptions. com

Sequencing of Therapies in Relapsed/Refractory FL and Novel Approaches on the Horizon

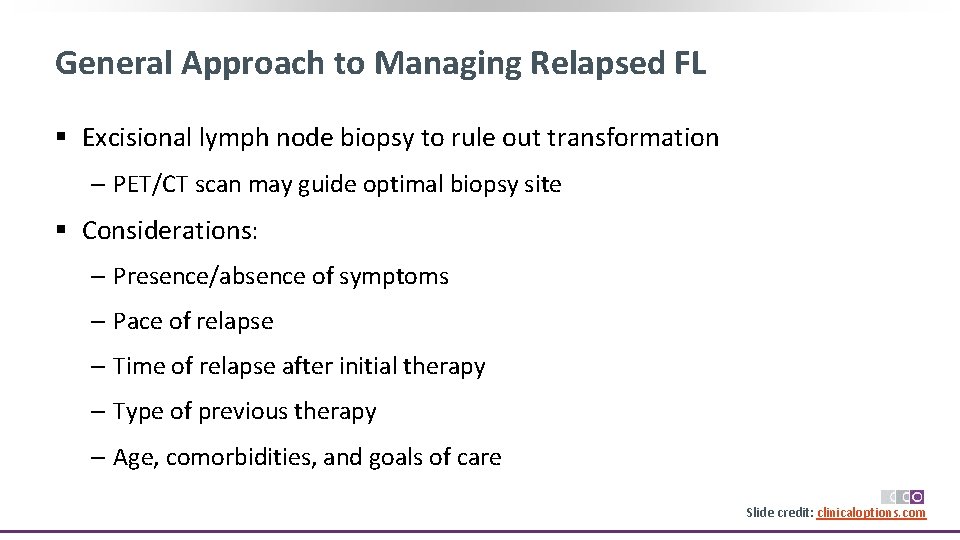
General Approach to Managing Relapsed FL § Excisional lymph node biopsy to rule out transformation ‒ PET/CT scan may guide optimal biopsy site § Considerations: ‒ Presence/absence of symptoms ‒ Pace of relapse ‒ Time of relapse after initial therapy ‒ Type of previous therapy ‒ Age, comorbidities, and goals of care Slide credit: clinicaloptions. com

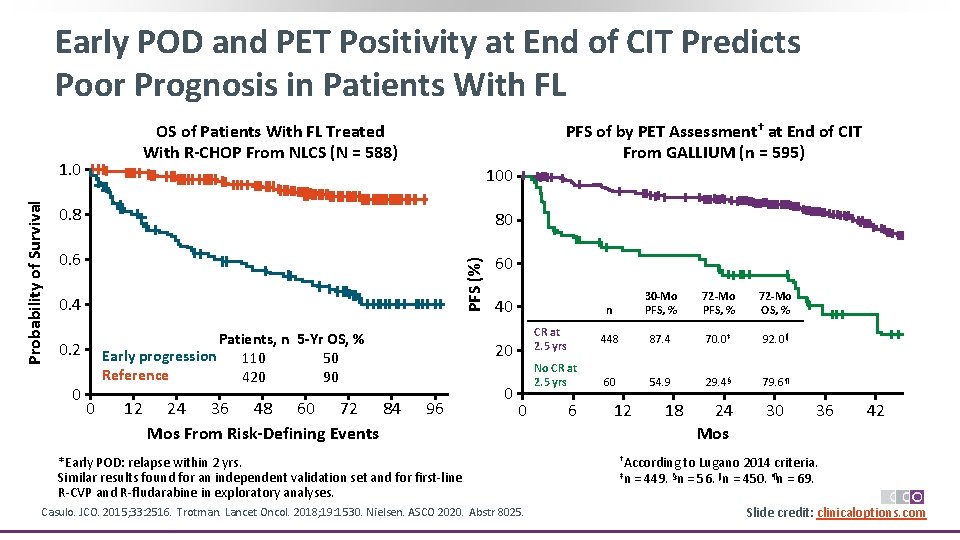
Early POD and PET Positivity at End of CIT Predicts Poor Prognosis in Patients With FL OS of Patients With FL Treated With R-CHOP From NLCS (N = 588) 100 0. 8 80 0. 6 60 PFS (%) Probability of Survival 1. 0 PFS of by PET Assessment† at End of CIT From GALLIUM (n = 595) 0. 4 Patients, n 5 -Yr OS, % Early progression 110 50 Reference 420 90 0. 2 0 0 12 24 36 48 60 72 84 Mos From Risk-Defining Events 40 CR at 2. 5 yrs 20 96 0 No CR at 2. 5 yrs 0 *Early POD: relapse within 2 yrs. Similar results found for an independent validation set and for first‐line R‐CVP and R‐fludarabine in exploratory analyses. Casulo. JCO. 2015; 33: 2516. Trotman. Lancet Oncol. 2018; 19: 1530. Nielsen. ASCO 2020. Abstr 8025. 6 n 30 -Mo PFS, % 72 -Mo OS, % 448 87. 4 70. 0‡ 92. 0‖ 60 54. 9 29. 4§ 79. 6¶ 12 18 24 Mos 30 36 42 †According to Lugano 2014 criteria. ‡n = 449. §n = 56. ‖n = 450. ¶n = 69. Slide credit: clinicaloptions. com
![ASCT vs No ASCT for Early Progressing FL Study Casulo et al1 Manna et ASCT vs No ASCT for Early Progressing FL Study Casulo et al[1] Manna et](https://slidetodoc.com/presentation_image_h/0c8e0d12fcfbde4a8ffd2b11d8b94ff1/image-47.jpg)
ASCT vs No ASCT for Early Progressing FL Study Casulo et al[1] Manna et al[2] Jurunovic et al[3] NLCS and CIBMTR Calgary GLSG Patient population Failure to achieve at least a PR or early relapse ≤ 2 yrs on frontline rituximab‐based CIT Early relapse ≤ 2 yrs following frontline CIT Progressive, relapsed, or refractory disease ≤ 2 yrs on systemic frontline therapy* N 349 § ASCT cohort: 175 § Non‐ASCT cohort: 174 84 § ASCT cohort: 50 § Non‐ASCT cohort: 34 113 § ASCT cohort: 52 § Non‐ASCT cohort: 46† Patient cohorts 5 -Yr PFS, % 5 -Yr OS, % Not reported § ASCT cohort: 67% § Non‐ASCT cohort: 60% § P =. 16 1. Casulo. Biol Blood Marrow Transplant. 2018; 24: 1163. 2. Manna. Leuk Lymphoma. 2019; 60: 133. 3. Jurinovic. Biol Blood Marrow Transplant. 2018; 24: 1172. Not reported § ASCT cohort: 85% § Non‐ASCT cohort: 58% § P =. 001 § ASCT cohort: 51% § Non‐ASCT cohort: 19% § P <. 0001 § ASCT cohort: 77% § Non‐ASCT cohort: 59% § P =. 031 *At ≤ 65 yrs of age. †Excludes patients with cytoreduction failure. Slide credit: clinicaloptions. com

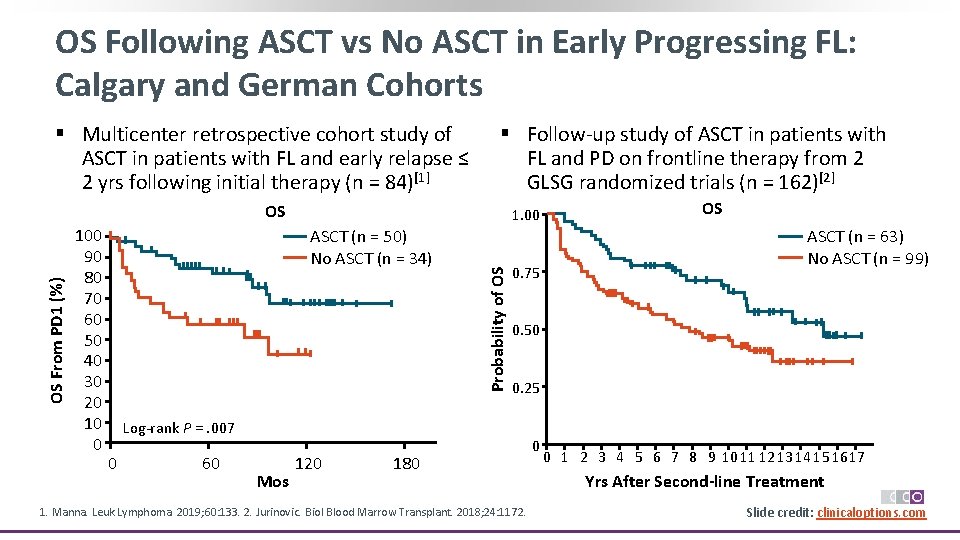
OS Following ASCT vs No ASCT in Early Progressing FL: Calgary and German Cohorts § Multicenter retrospective cohort study of ASCT in patients with FL and early relapse ≤ 2 yrs following initial therapy (n = 84)[1] § Follow‐up study of ASCT in patients with FL and PD on frontline therapy from 2 GLSG randomized trials (n = 162)[2] 100 90 80 70 60 50 40 30 20 10 0 1. 00 ASCT (n = 50) No ASCT (n = 34) Probability of OS OS From PD 1 (%) OS 0. 75 60 Mos 120 ASCT (n = 63) No ASCT (n = 99) 0. 50 0. 25 Log‐rank P =. 007 0 OS 180 1. Manna. Leuk Lymphoma. 2019; 60: 133. 2. Jurinovic. Biol Blood Marrow Transplant. 2018; 24: 1172. 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Yrs After Second-line Treatment Slide credit: clinicaloptions. com

GADOLIN: Bendamustine + Obinutuzumab and Maintenance Obinutuzumab in Rituximab-Refractory NHL Stratified by NHL subtype (FL vs other), Up to 6 prior therapies (≤ 2 vs > 2), refractory 28 -day cycles type, and geographic region Rituximab‐ refractory CD 20‐positive indolent NHL (N = 413) Obinutuzumab* + Bendamustine CR/ PR/ SD For 2 yrs or until PD Obinutuzumab maintenance 1000 mg IV Q 2 M Bendamustine *1000 mg IV on Days 1, 8, 15 cycle 1; Day 1 cycles 2‐ 6. Response monitored by CT scan post induction, then every 3 mos for 2 yrs, then every 6 mos (modified Cheson criteria 2007). § Primary endpoint: PFS assessed independently Sehn. Lancet Oncol. 2016; 17: 1081. Cheson. JCO. 2018; 36: 2259. Sehn. ASH 2019. Abstr 2822. Probability of OS § Randomized, open‐label, international phase III trial 1. 0 0. 8 0. 6 + ++ + OS in FL Cohort ++ + +++++++ + ++ 0. 4 0. 2 0 + Censored n ++++ ++ +++++ ++ +++++++++ +++++++ +++ m. PFS, m. TTNT, m. OS, ++ +++ + Mos Mos Benda + obin 164 24. 1 33. 6 NR Benda 171 13. 7 18. 0 60. 3 0 6 12 18 24 30 36 42 48 54 60 66 72 80 86 92 98104 Mos § Obinutuzumab + bendamustine followed by obinutuzumab is FDA approved for patients with FL who have relapsed after, or are refractory to, a rituximab‐containing regimen Slide credit: clinicaloptions. com

AUGMENT: Lenalidomide + Rituximab vs Placebo + Rituximab for R/R Indolent NHL § Multicenter, double‐blind, placebo‐controlled, randomized phase III trial Stratification for prior rituximab (yes vs no); time since last therapy (≤ 2 yrs vs > 2 yrs); FL vs MZL Patients with R/R grade 1‐ 3 a FL or MZL requiring treatment with prior use of ≥ 1 chemotherapy, immunotherapy, or CIT, and not refractory to rituximab (N = 358) Lenalidomide* + Rituximab (n = 178) Placebo* + Rituximab (n = 180) ≤ 12 cycles or until relapse, progression, or intolerability; 5 -yr follow-up for OS, secondary primary malignancies, subsequent therapy, histologic transformation *Anticoagulation or antiplatelet therapy recommended for patients at risk. Growth factor use in line with ASCO/ESMO guideline permitted. § Primary endpoint: IRC‐assessed PFS (2007 International Working Group criteria without PET) § Secondary endpoints: ORR, OS, histologic transformation, safety §Leonard. JCO. 2015; 33: 3635. Leonard. JCO. 2019; 37: 1188. Slide credit: clinicaloptions. com

AUGMENT: Efficacy and Safety Outcomes Probability of PFS IRC-Assessed PFS in Intention-to-Treat Population (Primary Endpoint)[1] 1. 0 0. 8 Lenalidomide + rituximab 0. 6 0. 4 0. 2 0 HR: 0. 46 (95% CI: 0. 34‐ 0. 62; P <. 0001) 0 Patients at Risk, n Len + rituximab 178 Placebo + rituximab 180 6 148 132 Placebo + rituximab 12 18 24 30 36 42 48 124 92 91 58 59 40 39 26 20 10 7 4 0 0 Mos Since Random Assignment R 2 (n = 176) R-Placebo (n = 180) Second primary malignancies 6 (3)* 10 (6)† Venous TE 6 (3) 3 (2) Arterial TE 1 (1) 4 (2) Mixed TE 3 (2) 1 (1) AEs of Interest, n (%) *n = 1 each, AML, carcinoid tumor of the GI tract, squamous cell carcinoma of the lung, basal cell carcinoma; n = 2, squamous cell carcinoma of the skin. †n = 1 each, adenocarcinoma of colon, malignant melanoma, papillary thyroid cancer, transitional cell cancer of the renal pelvis and ureter localized, squamous cell carcinoma of the skin; n = 2 each, AML, invasive ductal breast carcinoma, basal cell carcinoma. § Histologic transformation in 1% R 2 vs 6% R‐placebo, with an incidence/100 patient‐yrs of 0. 5 vs 2. 5, respectively Lenalidomide + rituximab is FDA approved for previously treated FL § OS improved with R 2 in patients with FL (HR: 0. 45; 95% CI: 0. 22‐ 0. 92; P =. 02) Leonard. JCO. 2019; 37: 1188. Lenalidomide PI. Slide credit: clinicaloptions. com

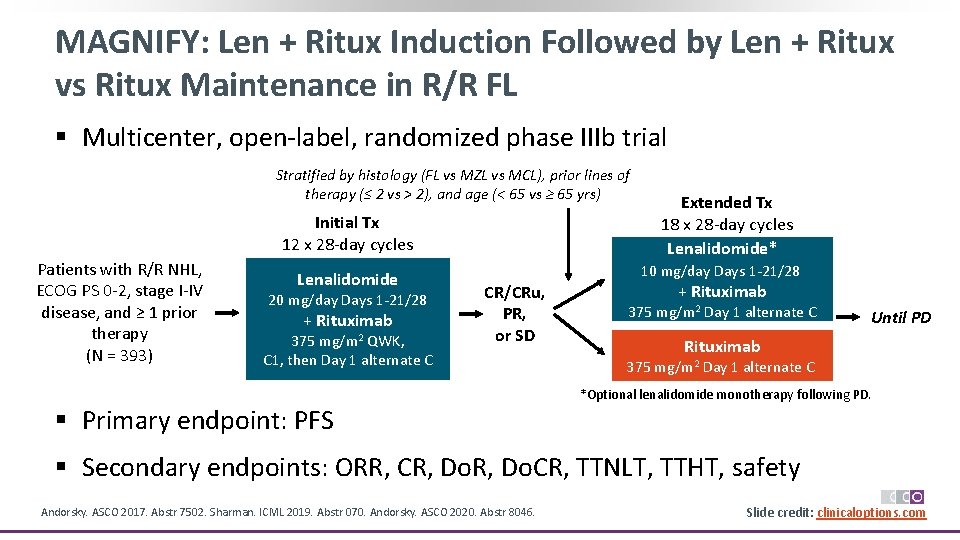
MAGNIFY: Len + Ritux Induction Followed by Len + Ritux vs Ritux Maintenance in R/R FL § Multicenter, open‐label, randomized phase IIIb trial Stratified by histology (FL vs MZL vs MCL), prior lines of therapy (≤ 2 vs > 2), and age (< 65 vs ≥ 65 yrs) Initial Tx 12 x 28‐day cycles Patients with R/R NHL, ECOG PS 0‐ 2, stage I‐IV disease, and ≥ 1 prior therapy (N = 393) Lenalidomide 20 mg/day Days 1‐ 21/28 + Rituximab 375 mg/m 2 QWK, C 1, then Day 1 alternate C Extended Tx 18 x 28‐day cycles Lenalidomide* 10 mg/day Days 1‐ 21/28 CR/CRu, PR, or SD + Rituximab 375 mg/m 2 Day 1 alternate C Until PD Rituximab 375 mg/m 2 Day 1 alternate C *Optional lenalidomide monotherapy following PD. § Primary endpoint: PFS § Secondary endpoints: ORR, CR, Do. CR, TTNLT, TTHT, safety Andorsky. ASCO 2017. Abstr 7502. Sharman. ICML 2019. Abstr 070. Andorsky. ASCO 2020. Abstr 8046. Slide credit: clinicaloptions. com

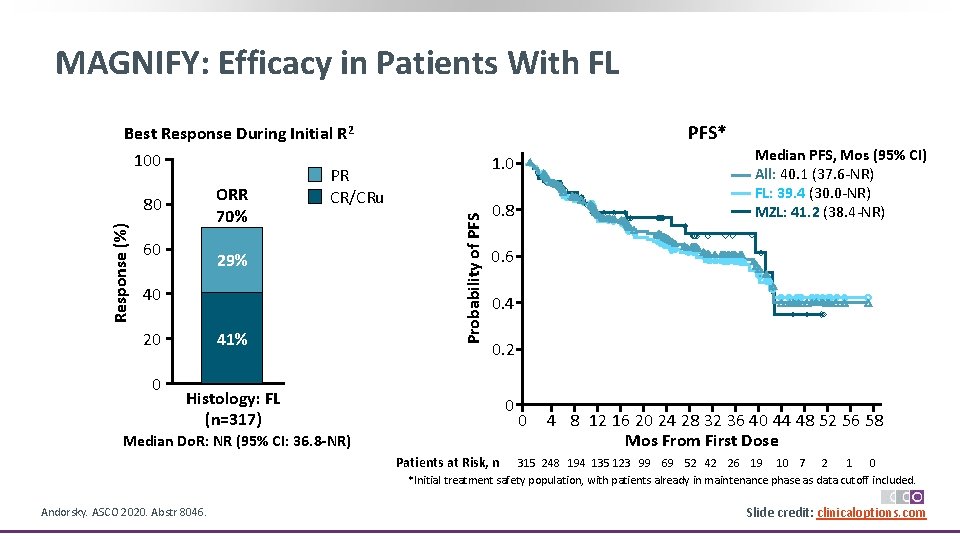
MAGNIFY: Efficacy in Patients With FL 60 29% 40 41% 20 0 PFS* 1. 0 Probability of PFS Response (%) Best Response During Initial R 2 100 PR ORR CR/CRu 80 70% 0. 8 Median PFS, Mos (95% CI) All: 40. 1 (37. 6‐NR) FL: 39. 4 (30. 0‐NR) MZL: 41. 2 (38. 4‐NR) 0. 6 0. 4 0. 2 Histology: FL (n=317) 0 Median Do. R: NR (95% CI: 36. 8 -NR) 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 58 Mos From First Dose Patients at Risk, n 315 248 194 135 123 99 69 52 42 26 19 10 7 2 1 0 *Initial treatment safety population, with patients already in maintenance phase as data cutoff included. Andorsky. ASCO 2020. Abstr 8046. Slide credit: clinicaloptions. com

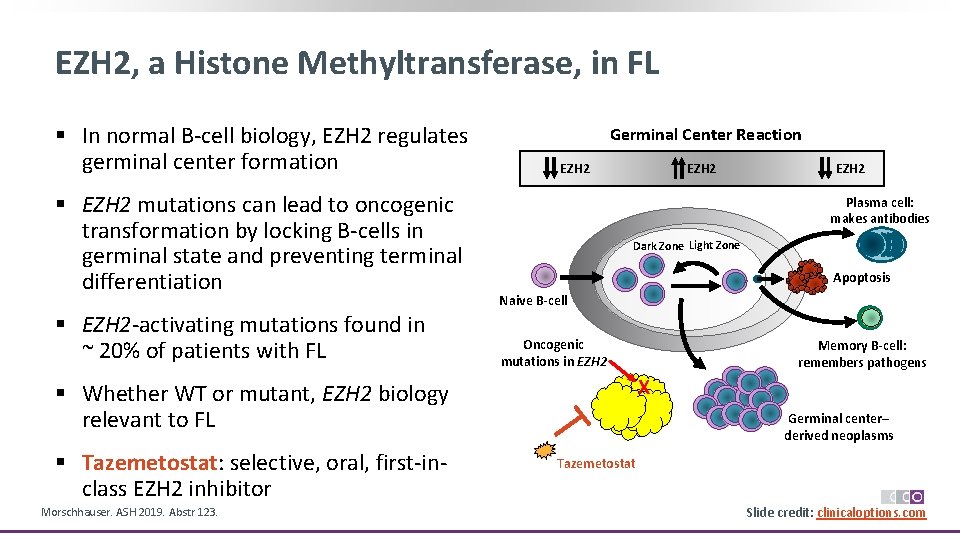
EZH 2, a Histone Methyltransferase, in FL § In normal B‐cell biology, EZH 2 regulates germinal center formation § EZH 2 mutations can lead to oncogenic transformation by locking B‐cells in germinal state and preventing terminal differentiation § EZH 2‐activating mutations found in ~ 20% of patients with FL Germinal Center Reaction EZH 2 Plasma cell: makes antibodies Dark Zone Light Zone Apoptosis Naive B-cell Oncogenic mutations in EZH 2 Morschhauser. ASH 2019. Abstr 123. Memory B-cell: remembers pathogens X § Whether WT or mutant, EZH 2 biology relevant to FL § Tazemetostat: selective, oral, first‐in‐ class EZH 2 inhibitor EZH 2 Germinal center– derived neoplasms Tazemetostat Slide credit: clinicaloptions. com

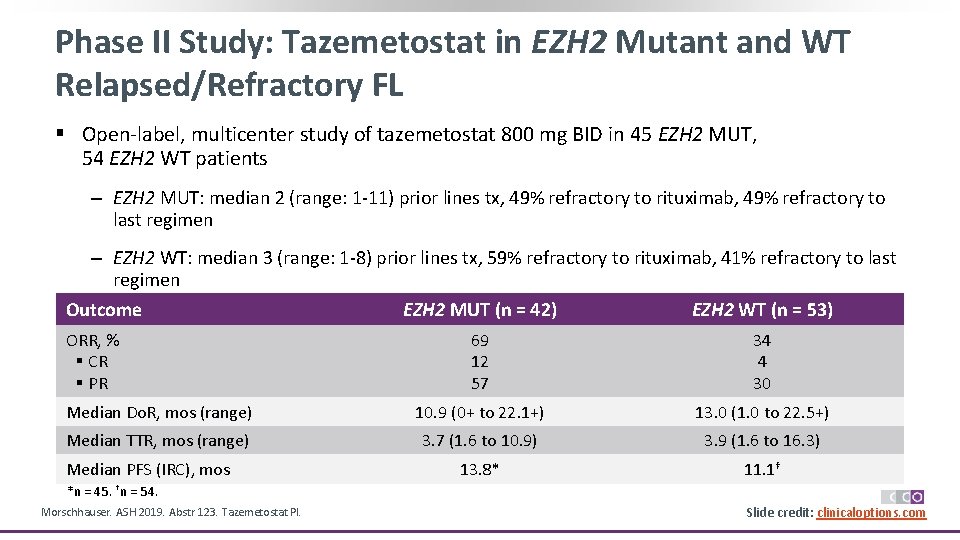
Phase II Study: Tazemetostat in EZH 2 Mutant and WT Relapsed/Refractory FL § Open‐label, multicenter study of tazemetostat 800 mg BID in 45 EZH 2 MUT, 54 EZH 2 WT patients ‒ EZH 2 MUT: median 2 (range: 1‐ 11) prior lines tx, 49% refractory to rituximab, 49% refractory to last regimen ‒ EZH 2 WT: median 3 (range: 1‐ 8) prior lines tx, 59% refractory to rituximab, 41% refractory to last regimen Outcome EZH 2 MUT (n = 42) EZH 2 WT (n = 53) ORR, % § CR § PR 69 12 57 34 4 30 Median Do. R, mos (range) 10. 9 (0+ to 22. 1+) 13. 0 (1. 0 to 22. 5+) Median TTR, mos (range) 3. 7 (1. 6 to 10. 9) 3. 9 (1. 6 to 16. 3) 13. 8* 11. 1† Median PFS (IRC), mos *n = 45. †n = 54. Morschhauser. ASH 2019. Abstr 123. Tazemetostat PI. Slide credit: clinicaloptions. com

Phase II Study: Tazemetostat Safety Treatment-Emergent AE, % Nausea Asthenia Diarrhea Fatigue Alopecia Cough Upper respiratory tract infection Bronchitis Anemia Abdominal pain Headache Vomiting Back pain Pyrexia Thrombocytopenia Morschhauser. ASH 2019. Abstr 123. N = 99 All 23 19 18 17 17 16 15 15 14 13 12 12 11 10 10 Grade ≥ 3 0 2 0 0 5 1 0 0 5 § Tazemetostat generally well tolerated, with low rate of grade ≥ 3 treatment‐related TEAEs ‒ 8% discontinued tx due to TEAEs ‒ 9% had dose reduction due to TEAEs ‒ 27% had dose interruption due to TEAEs § No treatment‐related deaths FDA approved for adults with EZH 2 mut+ R/R FL after ≥ 2 prior systemic therapies or any adult with R/R FL without alternative treatment options Slide credit: clinicaloptions. com
![PI 3 K Inhibitors Efficacy Agent Idelalisib1 Copanlisib2 Duvelisib3 DELTA CHRONOS 1 DYNAMO II PI 3 K Inhibitors: Efficacy Agent Idelalisib[1] Copanlisib[2] Duvelisib[3] DELTA CHRONOS‐ 1 DYNAMO II,](https://slidetodoc.com/presentation_image_h/0c8e0d12fcfbde4a8ffd2b11d8b94ff1/image-57.jpg)
PI 3 K Inhibitors: Efficacy Agent Idelalisib[1] Copanlisib[2] Duvelisib[3] DELTA CHRONOS‐ 1 DYNAMO II, single arm i. NHL with relapse ≤ 6 mos or refractory to rituximab and an alkylating agent i. NHL with relapse after or refractory to rituximab and an alkylating agent i. NHL with relapse ≤ 6 mos or refractory to rituximab and either CT or RIT N 125 i. NHL* 142 i. NHL†/104 FL 129 i. NHL‡/83 FL Median age, yrs (range) 64 (33‐ 87) 63 (25‐ 82) 65 (30‐ 90) Median lines tx (range) 4 (2‐ 12) 3 (2‐ 9) 3 (1‐ 18) 57 6 59/59 12/14 47/42 1/NR Median Do. R, mos 12. 5 23/12 10 Median PFS, mos 11 11. 2 9. 5 Trial Phase Population ORR, % § CR, % *Including FL, n = 72; SLL, n = 28; MZL, n = 15; LPL/WM, n = 10. †Including FL, n = 104; MZL, n = 23; SLL, n = 8; LPL/WM, n = 6; DLBCL, n = 1 (originally assessed as i. NHL). ‡Including FL, n = 83, SLL, n = 28; MZL, n = 18. 1. Gopal. NEJM. 2014; 370: 1008. 2. Dreyling. Am J Hematol. 2019; 95: 362. 3. Flinn. JCO. 2019; 37: 912. Slide credit: clinicaloptions. com
![PI 3 K Inhibitors Safety Agent Idelalisib1 2 Copanlisib3 4 Duvelisib5 6 delta alpha PI 3 K Inhibitors: Safety Agent Idelalisib[1, 2] Copanlisib[3, 4] Duvelisib[5, 6] delta alpha,](https://slidetodoc.com/presentation_image_h/0c8e0d12fcfbde4a8ffd2b11d8b94ff1/image-58.jpg)
PI 3 K Inhibitors: Safety Agent Idelalisib[1, 2] Copanlisib[3, 4] Duvelisib[5, 6] delta alpha, delta, gamma 150 mg orally BID 60 mg IV weekly (3 wks on, 1 wk off) 25 mg orally BID Grade ≥ 3 AE, % § ↓ ANC/PLT level § ALT/AST elevations § Diarrhea/colitis § Pneumonia § Hyperglycemia § Hypertension (n = 125) 27/6 13/8 13/4 7 ‐‐ ‐‐ (n = 142) 24/7 2/2 5/1 15 41 24 (n = 129) 25/12 5/3 15/5 5 ‐‐ ‐‐ Serious AEs of special interest Sepsis, opportunistic infections, diarrhea/colitis, cutaneous rxn, pneumonitis, hepatotoxicity, intestinal perforation, anaphylaxis Opportunistic infections, pneumonitis, diarrhea/colitis, cutaneous rxn, severe cutaneous rxn pneumonitis Monitoring LFTs, blood counts, signs of serious AEs, PJP infection, CMV reactivation/infection BP, blood sugar, blood counts, PJP infection, CMV reactivation/infection PI 3 K isoform target Dose/delivery 1. Gopal. NEJM. 2014; 370: 1008. 2. Idelalisib PI. 3. Dreyling. Am J Hematol 2019; 95: 362. 4. Copanlisib PI. 5. Flinn. JCO. 2019; 37: 912. 6. Duvelisib PI. LFTs, blood counts, signs of serious AEs, PJP infection, CMV reactivation/infection Slide credit: clinicaloptions. com

Considerations for PI 3 K Inhibitor Selection § All 3 FDA‐approved PI 3 K inhibitors have shown similar efficacy in the setting of relapsed/refractory FL § Different toxicity profiles may factor into choice of PI 3 K inhibitor, particularly in patients with comorbidities ‒ Hepatic toxicity and immune‐related colitis are the most clinically concerning with idelalisib and duvelisib, hyperglycemia and hypertension with copanlisib § Route of administration is another difference among PI 3 K inhibitors ‒ Idelalisib and duvelisib are taken orally; copanlisib is administered by IV § Choice of therapy should be individualized

Investigational PI 3 K Inhibitor Umbralisib in FL Best Chance In Target Lesions (%) Antitumor Response in Patients With FL (ITT) 50 25 0 § MTD not reached; 1 DLT in CLL cohort (grade 3 neutropenia at BL worsened to grade 4), no DLTs in B‐cell NHL cohort ‐ 25 ‐ 50 ‐ 75 ‐ 100 § Multicenter phase I/Ib trial assessing PI 3 K‐δ inhibitor umbralisib + anti‐CD 20 ublituximab in patients with R/R B‐cell NHL and CLL (N = 75, n = 19 with FL) Response, * n (%) ORR § CR § PR § SD § PD Do. R, mos (95% CI) FL (n = 18) 8 (44) 4 (22) 7 (39) 3 (17) 20. 3 (1. 7‐NR) *Comparable response data seen in an analysis of patients receiving umbralisib at ≥ therapeutic dose levels (1200 mg nonmicronized or > 600 mg micronized). Lunning. Blood. 2019; 134: 1811. § Most AEs were low grade ‒ Most common any‐grade AEs: diarrhea (in 71% of patients with indolent B‐cell NHL), nausea (63%), fatigue (4%) § Grade ≥ 3 AEs: diarrhea (8%), pneumonia (8%), hepatic toxicity (4%) Slide credit: clinicaloptions. com

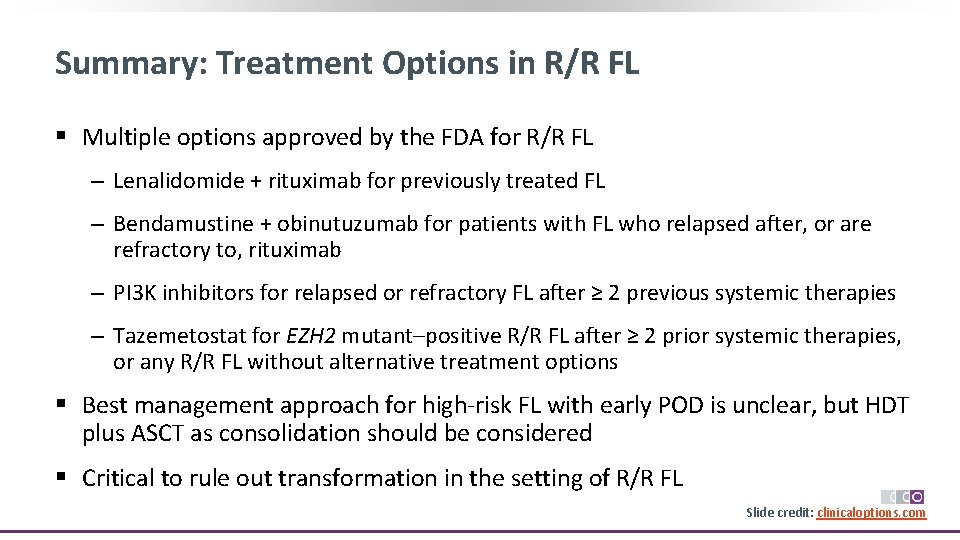
Summary: Treatment Options in R/R FL § Multiple options approved by the FDA for R/R FL ‒ Lenalidomide + rituximab for previously treated FL ‒ Bendamustine + obinutuzumab for patients with FL who relapsed after, or are refractory to, rituximab ‒ PI 3 K inhibitors for relapsed or refractory FL after ≥ 2 previous systemic therapies ‒ Tazemetostat for EZH 2 mutant–positive R/R FL after ≥ 2 prior systemic therapies, or any R/R FL without alternative treatment options § Best management approach for high‐risk FL with early POD is unclear, but HDT plus ASCT as consolidation should be considered § Critical to rule out transformation in the setting of R/R FL Slide credit: clinicaloptions. com

Novel Approaches on the Horizon for R/R FL

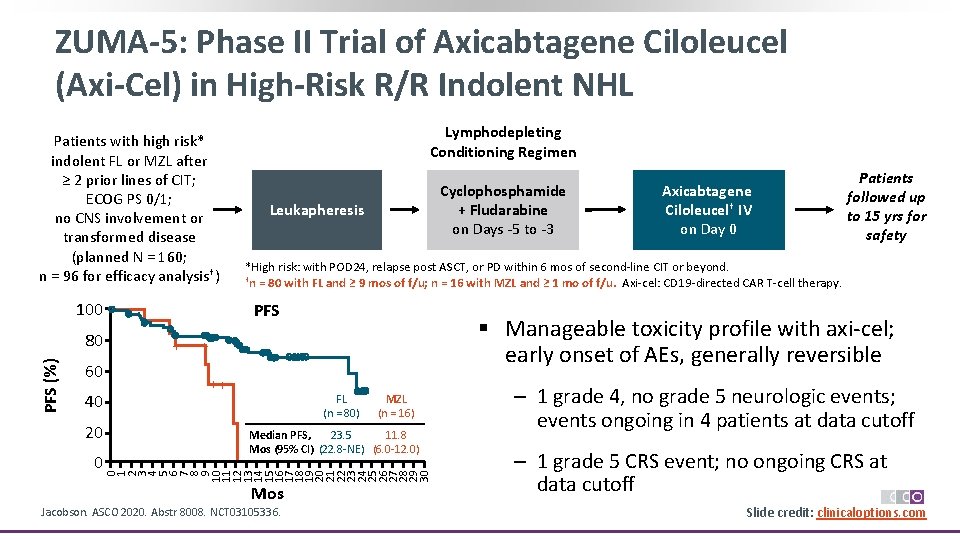
ZUMA-5: Phase II Trial of Axicabtagene Ciloleucel (Axi-Cel) in High-Risk R/R Indolent NHL Patients with high risk* indolent FL or MZL after ≥ 2 prior lines of CIT; ECOG PS 0/1; no CNS involvement or transformed disease (planned N = 160; n = 96 for efficacy analysis†) 100 60 40 20 0 Cyclophosphamide + Fludarabine on Days ‐ 5 to ‐ 3 Leukapheresis PFS + Axicabtagene Ciloleucel† IV on Day 0 Patients followed up to 15 yrs for safety *High risk: with POD 24, relapse post ASCT, or PD within 6 mos of second‐line CIT or beyond. †n = 80 with FL and ≥ 9 mos of f/u; n = 16 with MZL and ≥ 1 mo of f/u. Axi‐cel: CD 19‐directed CAR T‐cell therapy. § Manageable toxicity profile with axi‐cel; early onset of AEs, generally reversible + ++ FL (n = 80) MZL (n = 16) 23. 5 11. 8 Median PFS, Mos (95% CI) (22. 8‐NE) (6. 0‐ 12. 0) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 PFS (%) 80 Lymphodepleting Conditioning Regimen Mos Jacobson. ASCO 2020. Abstr 8008. NCT 03105336. ‒ 1 grade 4, no grade 5 neurologic events; events ongoing in 4 patients at data cutoff ‒ 1 grade 5 CRS event; no ongoing CRS at data cutoff Slide credit: clinicaloptions. com

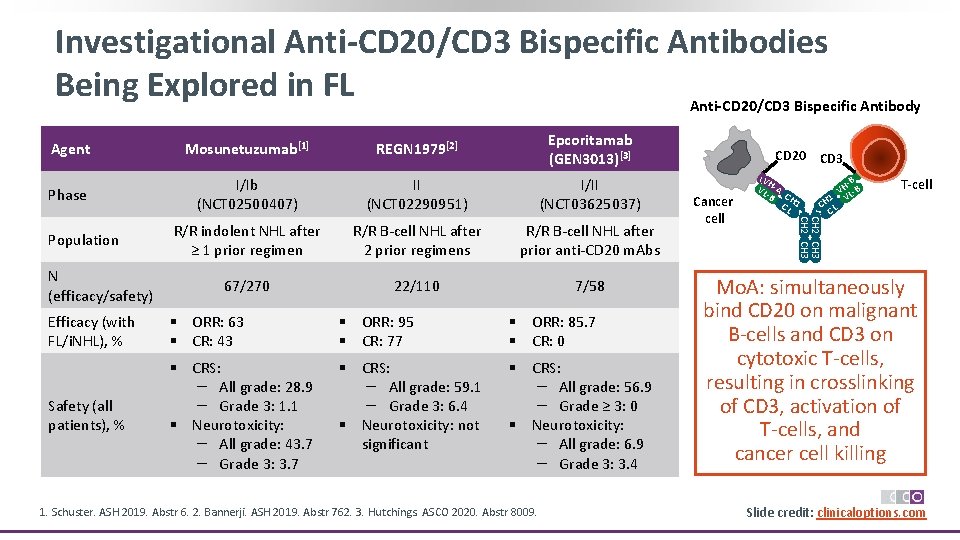
Investigational Anti-CD 20/CD 3 Bispecific Antibodies Being Explored in FL Anti-CD 20/CD 3 Bispecific Antibody Mosunetuzumab[1] REGN 1979[2] Epcoritamab (GEN 3013)[3] Phase I/Ib (NCT 02500407) II (NCT 02290951) I/II (NCT 03625037) R/R indolent NHL after ≥ 1 prior regimen R/R B‐cell NHL after 2 prior regimens R/R B‐cell NHL after prior anti‐CD 20 m. Abs 67/270 22/110 7/58 Population N (efficacy/safety) Efficacy (with FL/i. NHL), % § ORR: 63 § CR: 43 § ORR: 95 § CR: 77 § ORR: 85. 7 § CR: 0 Safety (all patients), % § CRS: – All grade: 28. 9 – Grade 3: 1. 1 § Neurotoxicity: – All grade: 43. 7 – Grade 3: 3. 7 § CRS: – All grade: 59. 1 – Grade 3: 6. 4 § Neurotoxicity: not significant § CRS: – All grade: 56. 9 – Grade ≥ 3: 0 § Neurotoxicity: – All grade: 6. 9 – Grade 3: 3. 4 1. Schuster. ASH 2019. Abstr 6. 2. Bannerji. ASH 2019. Abstr 762. 3. Hutchings. ASCO 2020. Abstr 8009. CD 20 CD 3 Cancer cell VH VL ‐A ‐B CH CL 1 ‐B VH L‐B 1 V CH L C T-cell CH 2 CH 3 Agent Mo. A: simultaneously bind CD 20 on malignant B‐cells and CD 3 on cytotoxic T‐cells, resulting in crosslinking of CD 3, activation of T‐cells, and cancer cell killing Slide credit: clinicaloptions. com

Activity of CD 47 Blockade + Rituximab in FL ‒ 5 F 9 acts synergistically with rituximab to augment macrophage‐mediated antibody‐ dependent phagocytosis of B‐cell NHL cells Best Overall Change in Size of Tumor Target Lesions 180 * 160 140 120 100 80 60 40 20 0 ‐ 20 ‐ 40 PR ‐ 60 ‐ 80 ‐ 100 Percent Change From Baseline § Phase Ib trial assessing safety, antitumor activity of investigational anti‐CD 47 antibody Hu 5 F 9‐G 4 (5 F 9) + rituximab in patients with R/R DLBCL or R/R FL (N = 22, n = 7 with FL) § Grade ≥ 3 AEs included insomnia, hypercalcemia, myelosuppression, muscular weakness, dyspnea, headache, IRR, pyrexia, chills Advani. NEJM. 2018; 379: 1711. * * * PD * * * * * 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 § Most AEs were low grade; DLTs rare ‒ Most common any‐grade AEs: chills (41%), headache (41%), anemia (41%), IRR (36%) 5 F 9 maintenance dose 10 mg/kg 20 mg/kg 30 mg/kg Patient No. All Patients (N = 22) DLBCL (n = 15) FL (n = 7) Objective response § CR § PR § SD § PD 11 (50) 8 (36) 3 (14) 8 (36) 6 (40) 5 (33) 1 (7) 3 (20) 6 (40) 5 (71) 3 (43) 2 (29) 0 2 (29) DCR 14 (64) 9 (60) 5 (71) Response, n (%) *Indicates patient with DLBCL; the remaining have FL. Slide credit: clinicaloptions. com

Conclusions: FL Moving Forward § Outcomes of FL have improved significantly, with most patients doing well on conventional therapy ‒ Median OS for advanced stage disease exceeds 20 yrs § Multiple options approved for relapsed/refractory disease ‒ Lenalidomide + rituximab, bendamustine + obinutuzumab, PI 3 Ki, tazemetostat § Subset of patients with poor outcomes identified clinically by early disease progression after chemoimmunotherapy ‒ Rationally defined, novel targeted agents (eg, CAR T‐cell therapy, bispecific Ab) will have biggest impact in this high‐risk group ‒ HDT + ASCT should be considered in these patients Slide credit: clinicaloptions. com

Go Online for More CCO Coverage of Follicular Lymphoma! CME/CE-certified text module with associated downloadable slideset Expert-authored commentaries on clinical challenges in FL management Downloadable Clinical Resource PDF with an FL management algorithm and toxicity management guide FL Interactive Decision Support Tool featuring the latest management recommendations from 5 lymphoma cancer experts (Update Coming Soon!) clinicaloptions. com/oncology
 Follicular nonhodgkins lymphoma
Follicular nonhodgkins lymphoma Unilateral balanced occlusion
Unilateral balanced occlusion Direct and indirect guidance examples
Direct and indirect guidance examples Papillary thyroid carcinoma gross
Papillary thyroid carcinoma gross Thyroid gland
Thyroid gland Thyroid follicular cells
Thyroid follicular cells Tonsil
Tonsil Pheochromocytoma
Pheochromocytoma Psammoma bodies
Psammoma bodies Melatonin histology
Melatonin histology Follicular carcinoma of thyroid
Follicular carcinoma of thyroid Principal cells location
Principal cells location Hormone
Hormone Follicular cells of thyroid gland
Follicular cells of thyroid gland Rickettsia microbiology pdf
Rickettsia microbiology pdf Dendritiform
Dendritiform Oogenesis and follicular development
Oogenesis and follicular development Female anatomy
Female anatomy Ameloblastoma follicular
Ameloblastoma follicular Follicular development diagram
Follicular development diagram Follicular study
Follicular study Follicular phase
Follicular phase How is economizing different from optimizing?
How is economizing different from optimizing? Cuda parallel reduction
Cuda parallel reduction Cuda parallel reduction
Cuda parallel reduction The fortran optimizing compiler
The fortran optimizing compiler Optimizing patient flow
Optimizing patient flow Burkitt lymphoma
Burkitt lymphoma Indolent non-hodgkin lymphoma quizlet
Indolent non-hodgkin lymphoma quizlet Difference between hodgkin and non hodgkin lymphoma
Difference between hodgkin and non hodgkin lymphoma What does lymphoma lump look like
What does lymphoma lump look like Hodgkin's lymphoma classification
Hodgkin's lymphoma classification Hodgkin's lymphoma alcohol pain
Hodgkin's lymphoma alcohol pain Hodgkin's lymphoma clinical presentation
Hodgkin's lymphoma clinical presentation Hodgkin lymphoma treatment
Hodgkin lymphoma treatment Leukemia vs lymphoma
Leukemia vs lymphoma Chylous ascites lymphoma
Chylous ascites lymphoma Copanlisib package insert
Copanlisib package insert Classification of hodgkin lymphoma
Classification of hodgkin lymphoma Primary cutaneous gamma/delta t-cell lymphoma
Primary cutaneous gamma/delta t-cell lymphoma Burkitt lymphoma
Burkitt lymphoma Hodjkins
Hodjkins Burkitt lymphoma
Burkitt lymphoma Szóbajön
Szóbajön Non-hodgkin lymphoma
Non-hodgkin lymphoma Hematological malignancies
Hematological malignancies Maintenance rituximab mantle cell lymphoma
Maintenance rituximab mantle cell lymphoma Hepatosplenic t-cell lymphoma
Hepatosplenic t-cell lymphoma Neoplastic proliferation of white blood cells
Neoplastic proliferation of white blood cells Marginális zóna lymphoma
Marginális zóna lymphoma Ann arbour staging system
Ann arbour staging system Crab criteria multiple myeloma
Crab criteria multiple myeloma Hervé tilly
Hervé tilly Burkitt lymphoma cytology
Burkitt lymphoma cytology Luis fayad
Luis fayad Thomas hodgkin
Thomas hodgkin Practical operations management
Practical operations management Practical investment management
Practical investment management Angelo kinicki management: a practical introduction
Angelo kinicki management: a practical introduction Read management: a practical introduction online
Read management: a practical introduction online Practical financial management
Practical financial management Management: a practical introduction
Management: a practical introduction Management a practical introduction 3e
Management a practical introduction 3e Management a practical introduction
Management a practical introduction Global force management implementation guidance
Global force management implementation guidance Pyramid levels of management
Pyramid levels of management Management pyramid
Management pyramid Top management middle management first line management
Top management middle management first line management