Practical Course ELISA Experiment to prove a HIV












- Slides: 24

Practical Course ELISA Experiment to prove a HIV infection proof of anti-HIV antibodies in blood

Preparations by the teacher

1. Preparation of buffer solutions • PBS-buffer (100 ml) 90 ml H 2 Odest. + 10 ml PBS (10 x) PBS-buffer is needed for rehydration of hydrophilic antigens and antibodies. • Washing buffer (900 ml) 805 ml H 2 Odest. + 90 ml PBS (10 x) + 4, 5 ml Tween 20 washing buffer is needed to dilute the antibody solution and for washing the microtiter plates. Tween 20 contains BSA to block free solid phase surface.

2. Rehydration ! • open the stopper of antibody and antigen vessels! • pipette 0, 5 ml diluted PSB-buffer to the antigen and to the primary and secondary antibodies! • close the vessels with the stopper and shake them for about 2 minutes! (stock solution) All stock solutions are fifty fold concentrated!

3. Labelling Label two polyethylene bottles with: - antigen - primary antibody - secondary antibody Antigen

Preparations by the pupils 8 teams The chemicals suffice for three courses with eight teams each! (3 x 160 µl stock solution = 480 µl; 500 µl (total volume)

Overview of the tubes tube color labelling contents quantity green “AG“ antigene 800 µl orange “s. AB“ 800 µl brown “SUB“ secondary enzyme linked antibody substrate violet “p. AB“ primary antibody 120 µl blue “WB“ washing buffer 120 µl yellow -- -- Still empty! 800 µl

1. Preparation of samples P 1 – P 8 - label the 8 yellow tubes with your group number! - pipet 60 µl primary antibody solution (p. AB, positive result of the test) or 60 µl washing buffer (WB, negative result of the test) into each of the 8 yellow tubes. Write down your setups! (P 1 – P 8 are serum samples)! ! - distribute 1 tube each of to the eight teams!

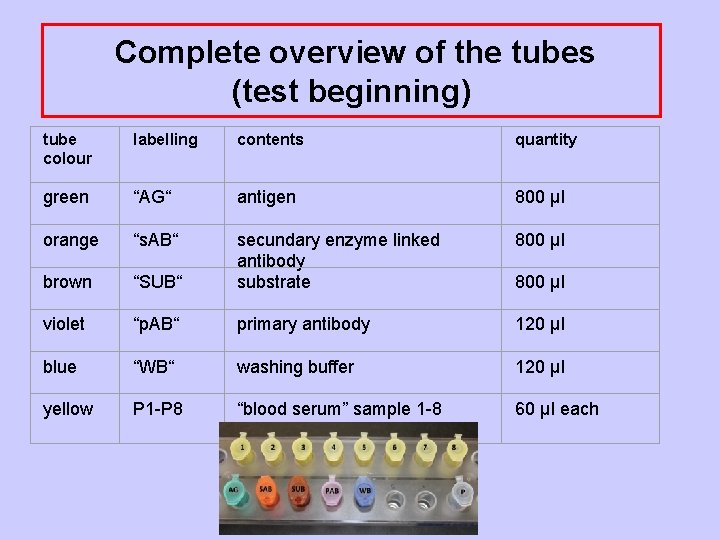
Complete overview of the tubes (test beginning) tube colour labelling contents quantity green “AG“ antigen 800 µl orange “s. AB“ 800 µl brown “SUB“ secundary enzyme linked antibody substrate violet “p. AB“ primary antibody 120 µl blue “WB“ washing buffer 120 µl yellow P 1 -P 8 “blood serum” sample 1 -8 60 µl each 800 µl

2. Execution of the test

2. 1 Antigen binding on microtiter plate - label a microtiter plate according to the following picture! + + - - P 1 P 2 P 3 P 4 P 5 P 6 P 7 P 8 - pipet 50 µl antigen solution (AG, green tube) into each of the 12 wells! - incubate for 5 minutes!

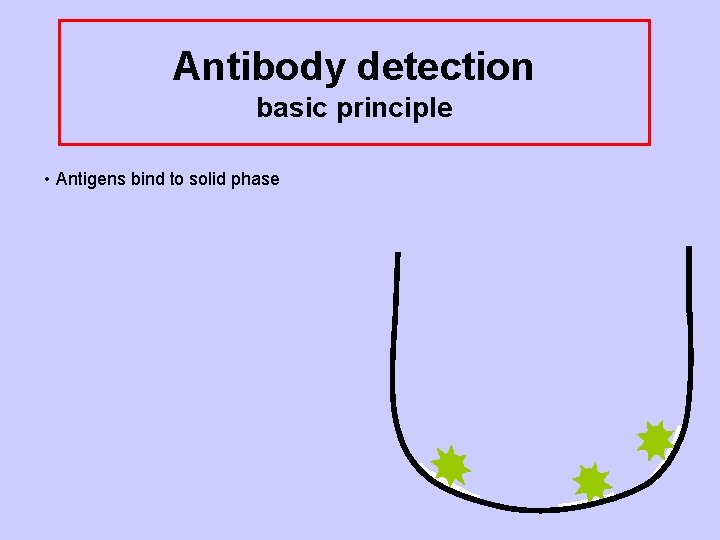
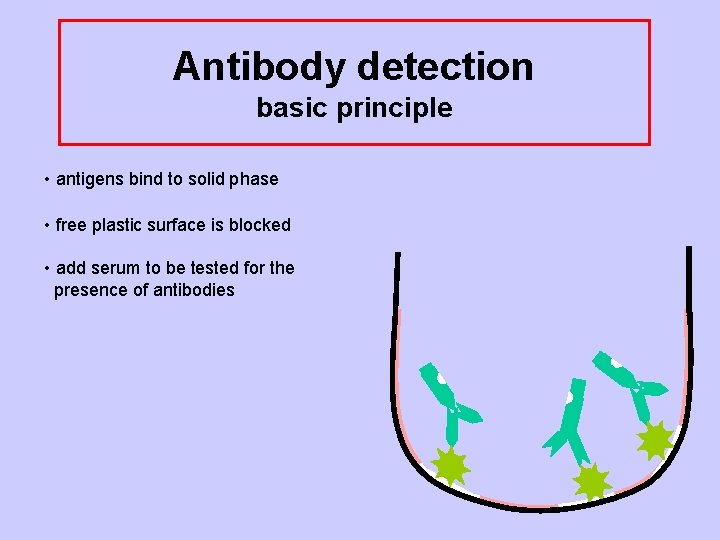
Antibody detection basic principle • Antigens bind to solid phase

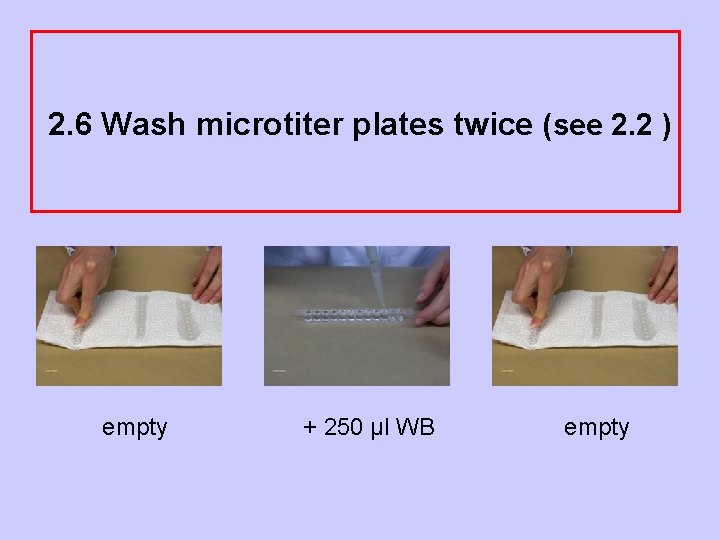
2. 2 Washing the microtiter plates • tap the plates on tissues! • pipet 250 µl washing buffer into each well! Attention! During pipeting the washing buffer should not spill over into other wells! • tap plates on new tissues!

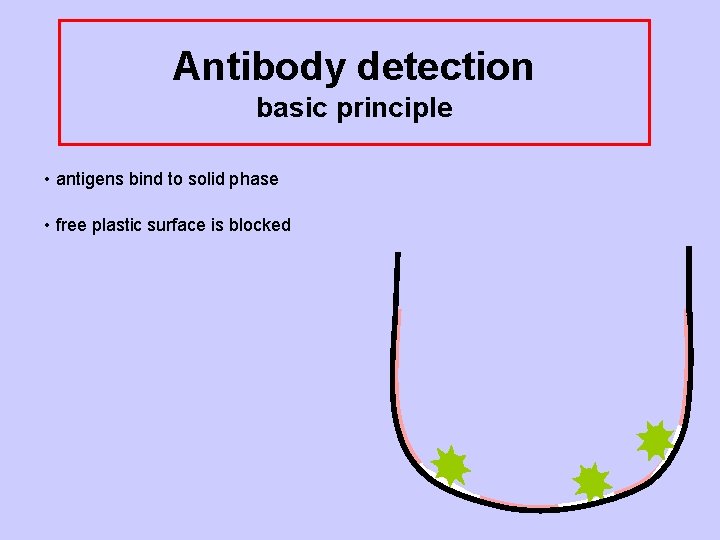
Antibody detection basic principle • antigens bind to solid phase • free plastic surface is blocked

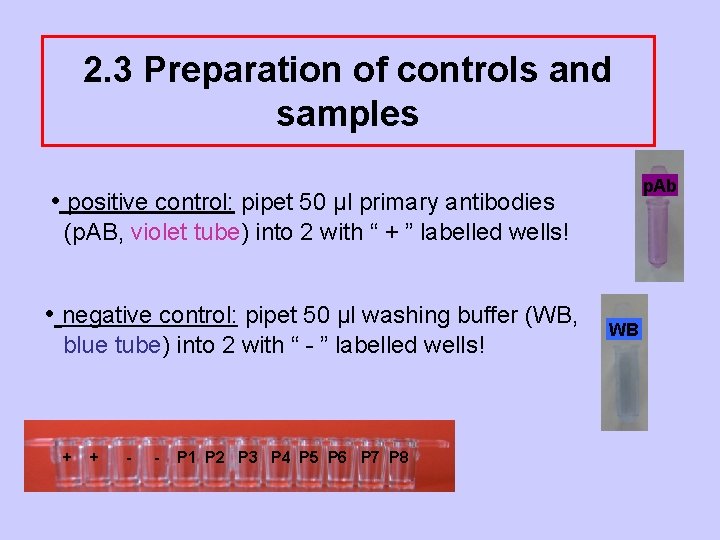
2. 3 Preparation of controls and samples p. Ab • positive control: pipet 50 µl primary antibodies (p. AB, violet tube) into 2 with “ + ” labelled wells! • negative control: pipet 50 µl washing buffer (WB, blue tube) into 2 with “ - ” labelled wells! + + - - P 1 P 2 P 3 P 4 P 5 P 6 P 7 P 8 WB

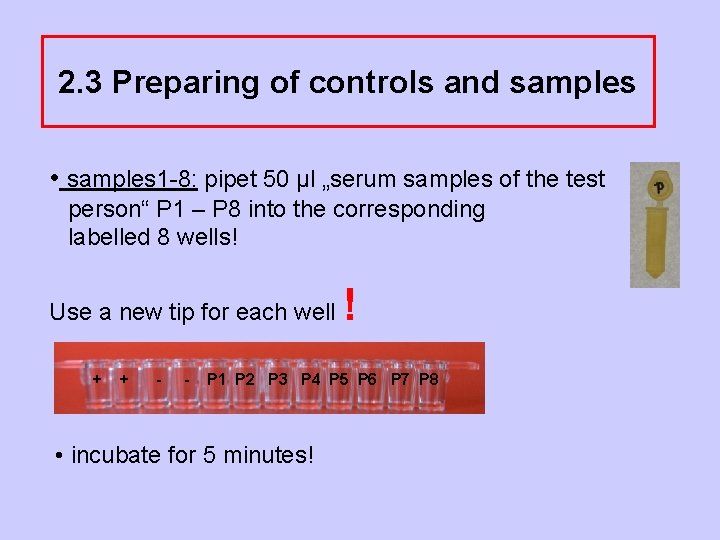
2. 3 Preparing of controls and samples • samples 1 -8: pipet 50 µl „serum samples of the test person“ P 1 – P 8 into the corresponding labelled 8 wells! Use a new tip for each well + + - - ! P 1 P 2 P 3 P 4 P 5 P 6 P 7 P 8 • incubate for 5 minutes!

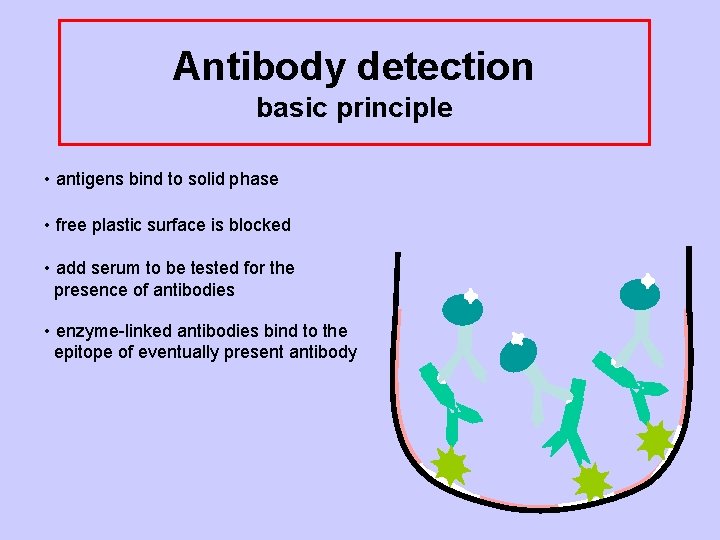
Antibody detection basic principle • antigens bind to solid phase • free plastic surface is blocked • add serum to be tested for the presence of antibodies

2. 4 Washing the microtiter plates (see 2. 2 ) empty + 250 µl WB empty

2. 5 Addition of secondary antibody • pipet 50 µl secondary antibody solution (s. AB, orange tube) in each of the 12 wells! • incubate for 5 minutes!

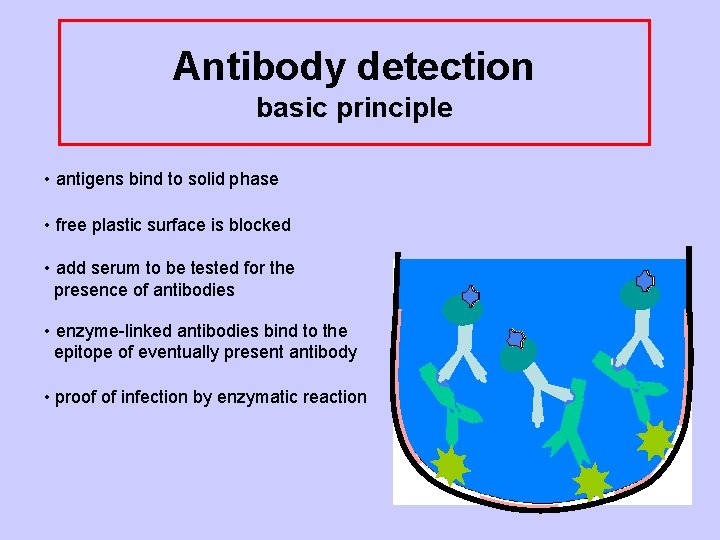
Antibody detection basic principle • antigens bind to solid phase • free plastic surface is blocked • add serum to be tested for the presence of antibodies • enzyme-linked antibodies bind to the epitope of eventually present antibody

2. 6 Wash microtiter plates twice (see 2. 2 ) empty + 250 µl WB empty

2. 7 Addition of the substrate • pipet 50 µl substrate solution (SUB, brown tube) into each of the 12 wells! • incubate for 5 minutes!

Antibody detection basic principle • antigens bind to solid phase • free plastic surface is blocked • add serum to be tested for the presence of antibodies • enzyme-linked antibodies bind to the epitope of eventually present antibody • proof of infection by enzymatic reaction

3. Evaluation Positive samples are blue-coloured, negative samples stay colourless (controls) Check your test results (P 1 – P 8) in the team! e. g. : + + - - P 1 P 2 P 3 P 4 P 5 P 6 P 7 P 8