Practical Blood Bank Lab 4 Weak D testing
Practical Blood Bank Lab 4 Weak D testing (Du)
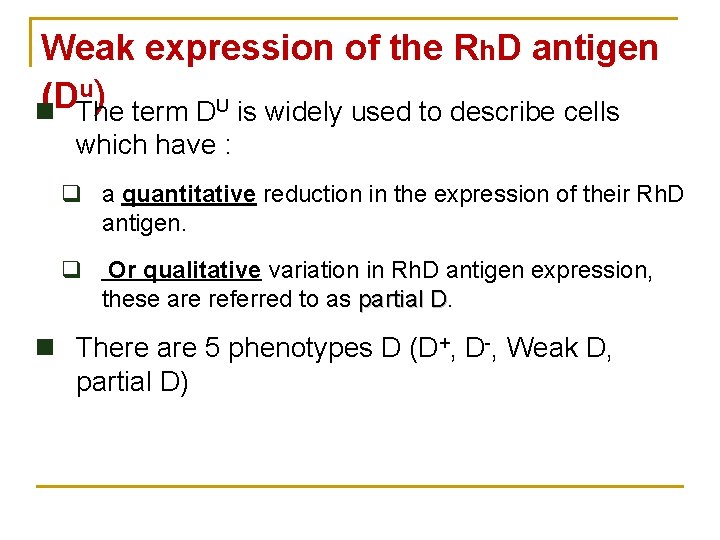
Weak expression of the Rh. D antigen u) (D n The term DU is widely used to describe cells which have : q a quantitative reduction in the expression of their Rh. D antigen. q Or qualitative variation in Rh. D antigen expression, these are referred to as partial D n There are 5 phenotypes D (D+, D-, Weak D, partial D)
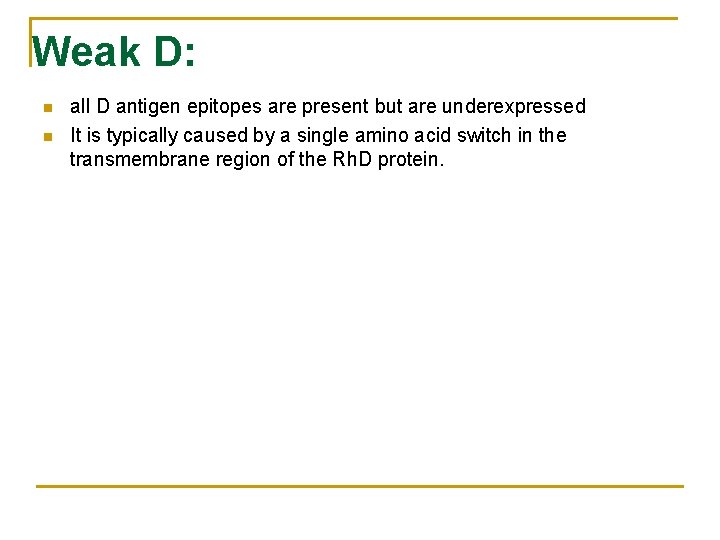
Weak D: n n all D antigen epitopes are present but are underexpressed It is typically caused by a single amino acid switch in the transmembrane region of the Rh. D protein.

Partial D n some D antigen epitopes are missing
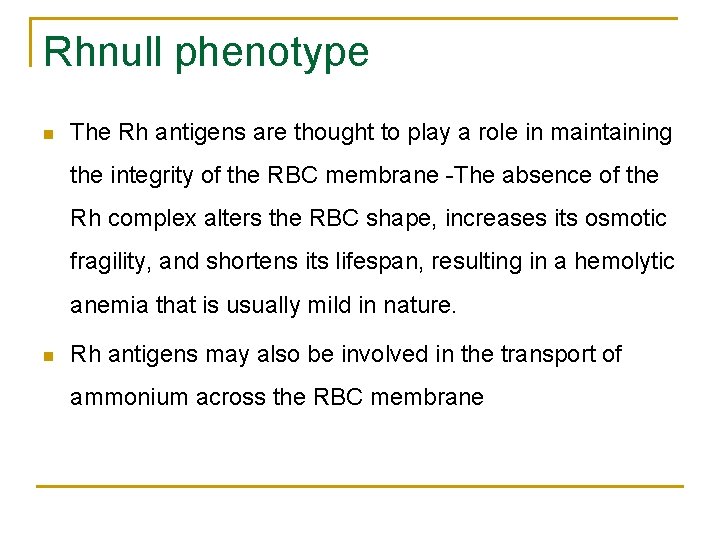
Rhnull phenotype n The Rh antigens are thought to play a role in maintaining the integrity of the RBC membrane -The absence of the Rh complex alters the RBC shape, increases its osmotic fragility, and shortens its lifespan, resulting in a hemolytic anemia that is usually mild in nature. n Rh antigens may also be involved in the transport of ammonium across the RBC membrane
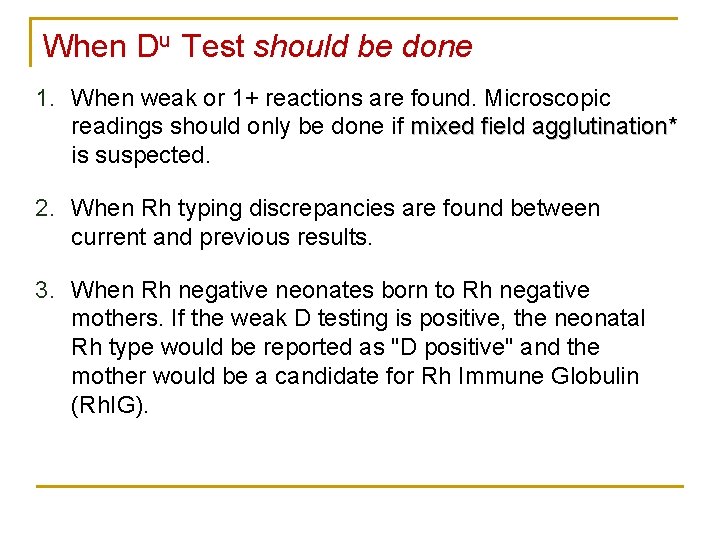
When Du Test should be done 1. When weak or 1+ reactions are found. Microscopic readings should only be done if mixed field agglutination* is suspected. 2. When Rh typing discrepancies are found between current and previous results. 3. When Rh negative neonates born to Rh negative mothers. If the weak D testing is positive, the neonatal Rh type would be reported as "D positive" and the mother would be a candidate for Rh Immune Globulin (Rh. IG).
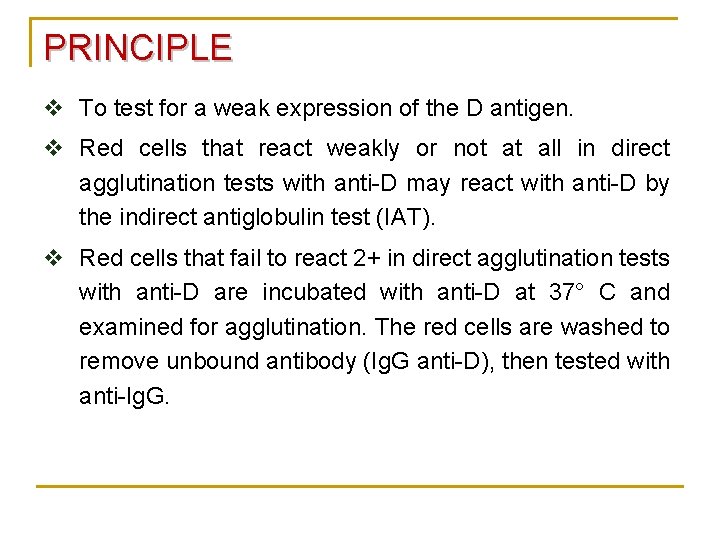
PRINCIPLE v To test for a weak expression of the D antigen. v Red cells that react weakly or not at all in direct agglutination tests with anti-D may react with anti-D by the indirect antiglobulin test (IAT). v Red cells that fail to react 2+ in direct agglutination tests with anti-D are incubated with anti-D at 37° C and examined for agglutination. The red cells are washed to remove unbound antibody (Ig. G anti-D), then tested with anti-Ig. G.
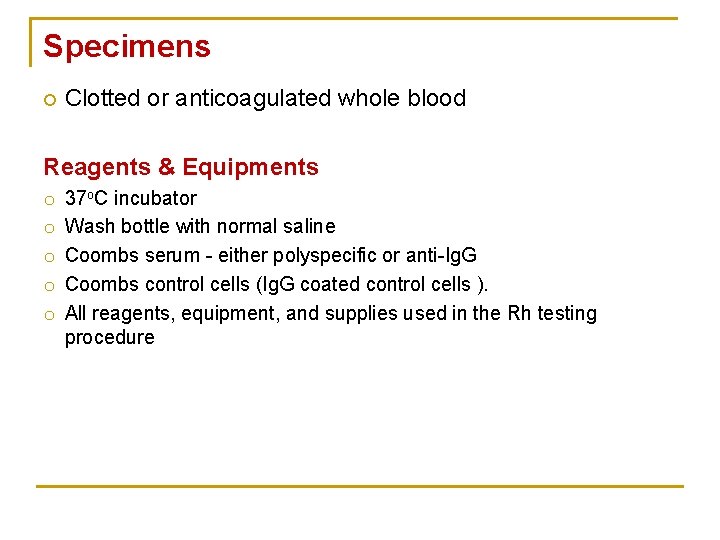
Specimens Clotted or anticoagulated whole blood Reagents & Equipments o o o 37 o. C incubator Wash bottle with normal saline Coombs serum - either polyspecific or anti-Ig. G Coombs control cells (Ig. G coated control cells ). All reagents, equipment, and supplies used in the Rh testing procedure

Recommended techniques SLIDE TECHNIQUE 1. Add to a clean, labeled slide: q One drop of anti-D Ig. M and Ig. G blend. q One drop of the test red cells suspension. 2. Mix well by gently and continuously rocking the slide for approx. 30 seconds and incubate the slide for 5 minutes at room temperature, with occasional mixing, we can put the slide on source of light as heat source (Box Lamp) 3. Examine macroscopically for agglutination. A diffuse light source may aid reading.
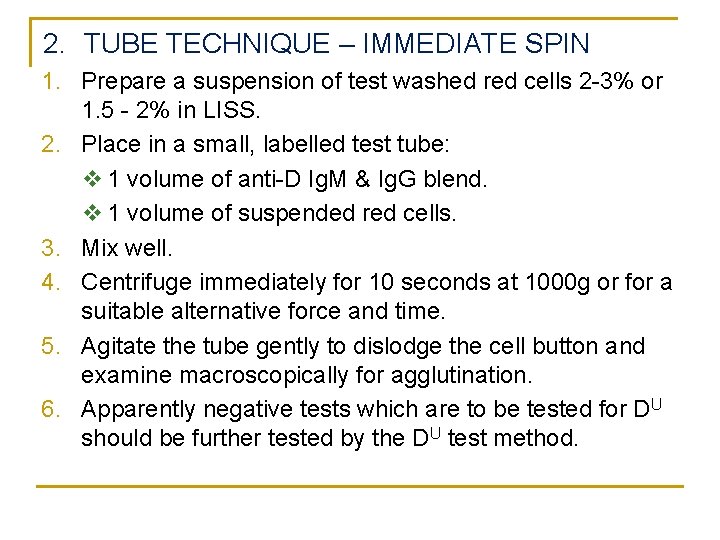
2. TUBE TECHNIQUE – IMMEDIATE SPIN 1. Prepare a suspension of test washed red cells 2 -3% or 1. 5 - 2% in LISS. 2. Place in a small, labelled test tube: v 1 volume of anti-D Ig. M & Ig. G blend. v 1 volume of suspended red cells. 3. Mix well. 4. Centrifuge immediately for 10 seconds at 1000 g or for a suitable alternative force and time. 5. Agitate the tube gently to dislodge the cell button and examine macroscopically for agglutination. 6. Apparently negative tests which are to be tested for DU should be further tested by the DU test method.
3. TUBE TECHNIQUE – LISS 1. Place in a small, labelled test tube: v 1 volume of Anti-D blood grouping reagent v 1 volume of red cells suspended 1. 5 -2% in LISS. 2. Mix well and incubate for 15 -20 minutes at 37°C. 3. Centrifuge immediately for 10 seconds at 1000 g or for a suitable alternative force and time. 4. Agitate the tube gently to dislodge the cell button and examine macroscopically for agglutination.
4. DU TEST METHOD (indirect antiglobulin test (IAT). ) After reading the immediate spin results, re-incubate the test for a further 20 minutes at 37°C before completing the DU test method described below. OR After reading the LISS tube test, complete the DU test, without further incubation, following the procedure given below.
DU TEST METHOD 1. Wash the test 4 times with a large excess of PBS (e. g. 4 ml of PBS per 12 (or 10) x 75 mm glass tubes). NOTE: Allow adequate spin time to sediment the red cells make sure that most of the residual saline is removed at the end if each wash to leave a ‘dry’ cell button. 2. Add two drops of anti-human globulin reagent to each tube. 3. Mix thoroughly. 4. Centrifuge at 1000 g for 10 seconds or for a suitable alternative force and time. 5. Agitate the tube gently to dislodge the cell button and examine macroscopically and microscopically for agglutination. 6. Add 1 drop of Ig. G-coated control cells to the tube(s) with negative results. Centrifuge, resuspend cells, read macroscopically and record results. Agglutination (2+) shall be present or the test shall be repeated.
Procedural Notes Tests should be read immediately after centrifugation. Delay may cause bound Ig. G to dissociate from red cells and either leave too little Ig. G to detect or neutralize AHG reagent causing false negative results. Interpretation A negative result in the immediate spin phase but agglutination in the D tube following incubation (with no agglutination in the DC tube) indicates a positive test for weak D. Lack of agglutination is a negative test and the patient is considered truly D negative. Agglutination in the DC tube invalidates the test.
- Slides: 14