Practical Blood Bank Compatibility Testing Blood Transfusion Process

Practical Blood Bank Compatibility Testing
Blood Transfusion Process Pre-transfusion Transfusion Post-transfusion
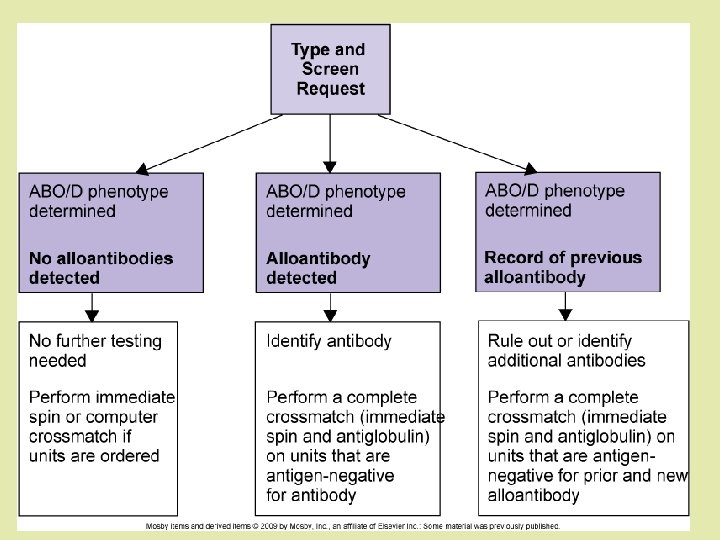
What is compatibility testing? Also called pretransfusion testing Purpose: To select blood components that will not cause harm to the recipient and will have acceptable survival when transfused If properly performed, compatibility tests will confirm ABO compatibility between the component and the recipient and will detect the most clinically significant unexpected antibodies
Compatibility testing? There are several components of compatibility testing Proper specimen collection Reviewing patient transfusion history ABO, Rh, and antibody testing (screen/ID) Crossmatching Actual transfusion

Compatibility testing Can be divided into 3 categories: Preanalytical procedures Serological testing Postanalytical procedures
Pre-analytical phases 1. 2. Patient identification Must confirm recipient’s ID from bracelet ON the patient Full patient name and hospital number Name of physician Review of patient history Look at recipient’s records for any prior unexpected antibodies Previous transfusion reactions
3. Specimen collection The sample should also have the full patient name, hospital number, and physician Date and time of collection, phlebotomist’s initials All of this should be on the request form and the sample Collected in tube with EDTA or no additives If the venipuncture causes hemolysis, the sample may be rejected If the sample is drawn from an IV line, the IV infusion should be stopped 5 -10 minutes prior to blood drawing and the first 10 m. L discarded Testing should be performed on samples less than 72 hours or else complement dependent antibodies may be missed (complement can become unstable)

Serological Testing 3 tests: ABO/Rh Antibody detection/identification Crossmatch

ABO/Rh Typing In the ABO typing, the forward and reverse MUST match In the Rh typing, the control must be negative Both of these will indicate what type of blood should be given
Antibody screen and/or ID The antibody screen will detect the presence of any unexpected antibodies in patient serum If antibodies are detected, identification should be performed using panel cells (with an autocontrol) IS 37° (LISS) AHG If an antibody is present, units negative for the antigen must be given Proceed to the crossmatch…
Crossmatching Purpose: Prevent transfusion reactions Increase in vivo survival of red cells Double checks for ABO errors Another method of detecting antibodies

Crossmatch Two types of crossmatches Major – routinely performed in labs Minor – not required by AABB since 1976
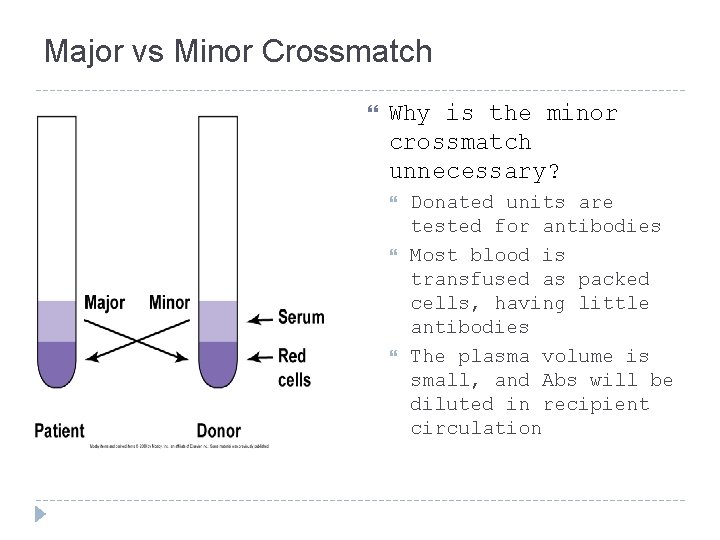
Major vs Minor Crossmatch Why is the minor crossmatch unnecessary? Donated units are tested for antibodies Most blood is transfused as packed cells, having little antibodies The plasma volume is small, and Abs will be diluted in recipient circulation

Crossmatches The crossmatch “shall use methods that demonstrate ABO incompatibility and clinically significant antibodies to red cell antigens and shall include an antiglobulin phase”
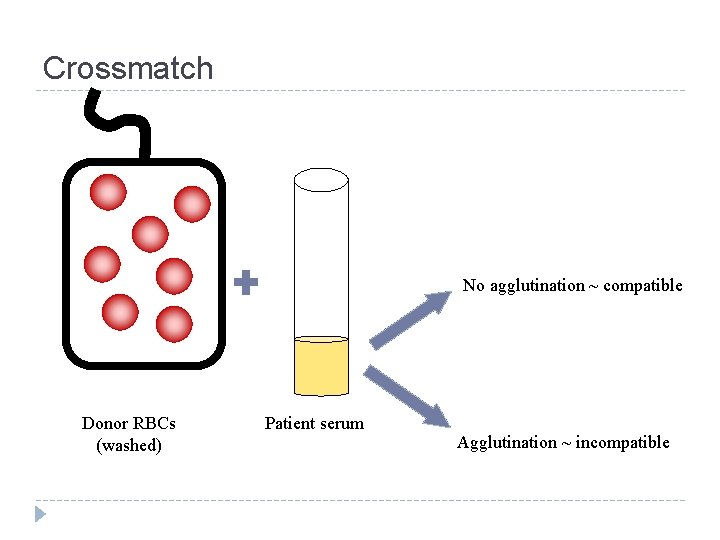
Crossmatch No agglutination ~ compatible Donor RBCs (washed) Patient serum Agglutination ~ incompatible
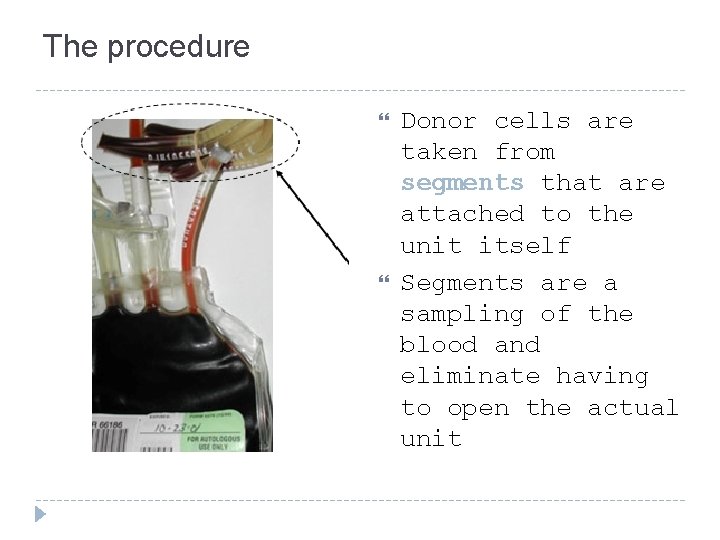
The procedure Donor cells are taken from segments that are attached to the unit itself Segments are a sampling of the blood and eliminate having to open the actual unit

Units of whole blood with segments attached
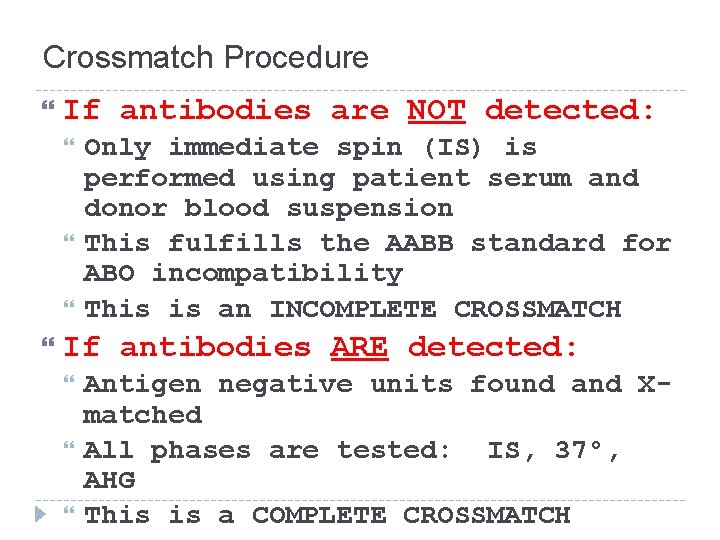
Crossmatch Procedure If antibodies are NOT detected: Only immediate spin (IS) is performed using patient serum and donor blood suspension This fulfills the AABB standard for ABO incompatibility This is an INCOMPLETE CROSSMATCH If antibodies ARE detected: Antigen negative units found and Xmatched All phases are tested: IS, 37°, AHG This is a COMPLETE CROSSMATCH
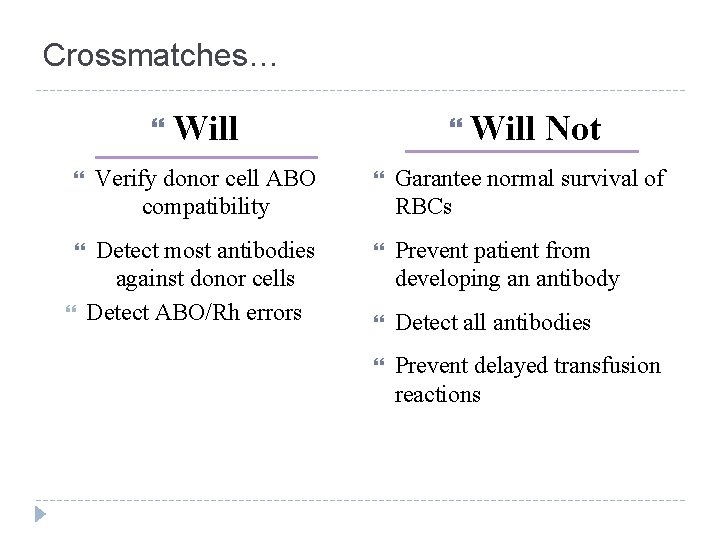
Crossmatches… Will Not Verify donor cell ABO compatibility Garantee normal survival of RBCs Detect most antibodies against donor cells Detect ABO/Rh errors Prevent patient from developing an antibody Detect all antibodies Prevent delayed transfusion reactions
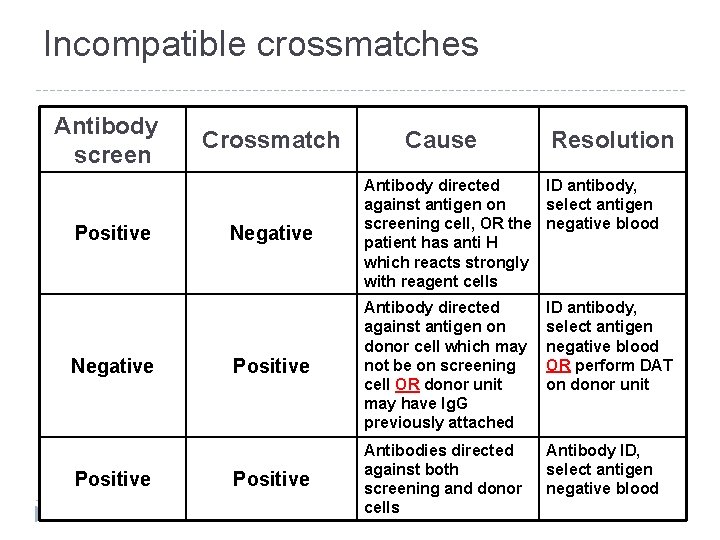
Incompatible crossmatches Antibody screen Positive Negative Positive Crossmatch Cause Resolution Negative Antibody directed ID antibody, against antigen on select antigen screening cell, OR the negative blood patient has anti H which reacts strongly with reagent cells Positive Antibody directed against antigen on donor cell which may not be on screening cell OR donor unit may have Ig. G previously attached ID antibody, select antigen negative blood OR perform DAT on donor unit Positive Antibodies directed against both screening and donor cells Antibody ID, select antigen negative blood
Additional Information on Types of Compatibility Tests Manual (IS and IAT) Gel Technology Electronic (Computerized) Cross match Red cell Affinity Column Technology (Re. ACT) Solid Phase Adherence Assays (SPAA)
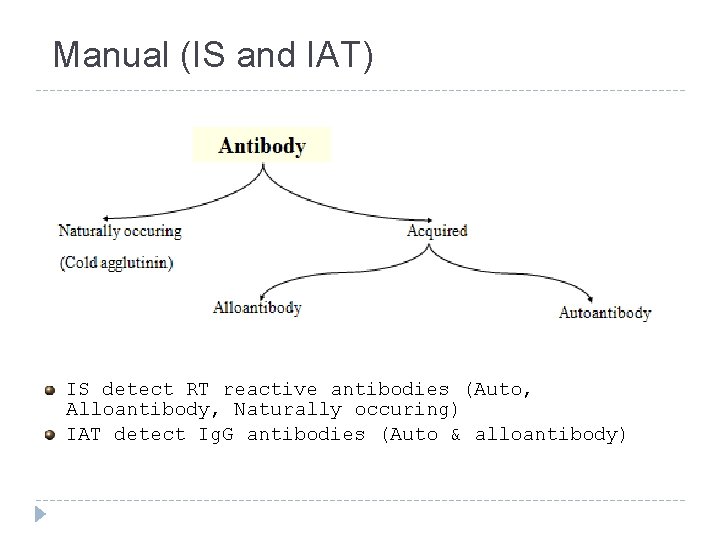
Manual (IS and IAT) IS detect RT reactive antibodies (Auto, Alloantibody, Naturally occuring) IAT detect Ig. G antibodies (Auto & alloantibody)
Gel Technology Patient serum, and 1% of suspended RBCs in LIM are dispensed into the microtube and incubated at 37 o. C for 15 minutes. The card containing the microtubes is then centrifuged at a controlled speed for 10 minutes. At the start of centrifugation the cells are separated from the serum; then they meet the AHG contained in the microtube. Finally the cells are trapped by the gel (if agglutinated) or pellet to the bottom of the tube.

Other methods for blood grouping Gel Cards containing Anti-A, Anti-B, and Anti-A, B are used to test patient or donor red blood cells for the presence or absence of the A and/or B antigens. The results of red blood cell grouping should be confirmed by reverse (serum) grouping, i. e. testing the individual’s serum with known A 1 and B red blood cells. In the Gel Test™, the specific antibody (Anti-A, Anti-B, or Anti-D) is incorporated into the gel. This gel has been pre-filled into the microtubes of the plastic card. As the red blood cells pass through the gel, they come in contact with the antibody. Red blood cells with the specific antigen will agglutinate when combined with the corresponding antibody in the gel during the centrifugation step.
Interpretation of Results A positive reaction is recorded when red cells are retained in or A positive reaction above the gel column after centrifugation A negative reaction is recorded when a distinct button of cells A negative reaction sediment to the bottom of the column after centrifugation. A positive reaction in the MTS Control microtube indicates a false the MTS Control positive reaction may have occurred in the corresponding blood grouping microtube, thus invalidating the blood grouping tests. Drying, discoloration, bubbles, crystals, other artifacts, opened or damaged seals may indicate product alteration A buffered gel suspension is contained in two (2) microtubes of the A/B/D Monoclonal and Reverse Grouping Card™. Sodium Azide (0. 1% final concentration) is added as a preservative.
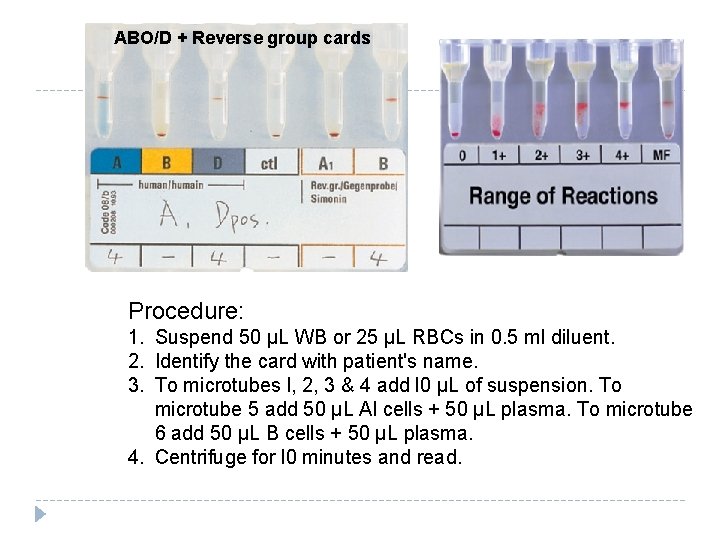
ABO/D + Reverse group cards Procedure: 1. Suspend 50 µL WB or 25 µL RBCs in 0. 5 ml diluent. 2. Identify the card with patient's name. 3. To microtubes l, 2, 3 & 4 add l 0 µL of suspension. To microtube 5 add 50 µL Al cells + 50 µL plasma. To microtube 6 add 50 µL B cells + 50 µL plasma. 4. Centrifuge for l 0 minutes and read.

New Technologies… The electronic crossmatch According to the AABB, the following must be fulfilled: Critical elements of the information system have been validated on-site. No clinically significant antibodies are detected in the current blood sample and there is no record of clinically significant antibodies in the past
Computer crossmatch (cont’d) The patient's ABO group and Rh type has been done twice and entered in the computer The donor ABO/Rh have been confirmed and entered in the computer. The donor unit identification number, component name, and ABO/Rh type must also be entered in the computer The computer system will alert the technologist to ABO & Rh discrepancies between information on the donor label and results of donor confirmatory testing
Red Cell Affinity Column Technology (Re. ACT) Based on affinity adherence of coated red cells in an immunologically active matrix. Antibody- sensitized red cells bind or adsorbed to ligands attached to an agarose matrix. The main ligand is Protein G (prepared from Group C or G Streptococcus or by recombinant technology), which has high affinity for all four Ig. G subclasses. Another Re. ACT ligand is Protein A (from Group A Staphlococcus), which binds to Ig. G 1, 2, and 4.
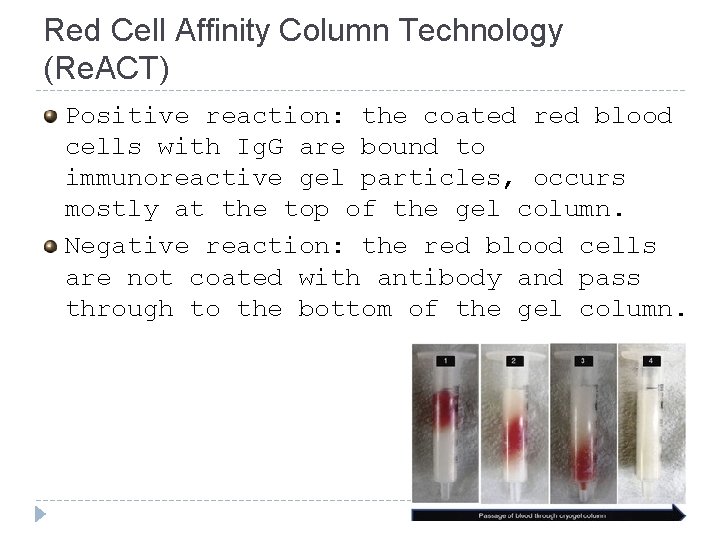
Red Cell Affinity Column Technology (Re. ACT) Positive reaction: the coated red blood cells with Ig. G are bound to immunoreactive gel particles, occurs mostly at the top of the gel column. Negative reaction: the red blood cells are not coated with antibody and pass through to the bottom of the gel column.
Solid Phase Adherence Assays (SPAA) Uses red cell membrane bound to the surfaces of polystyrene microtitration strip wells, capturing Ig. G antibodies (if present) in patient sera. Patient serum is added to wells coated with screen cells Incubated at 37 o. C for 15 min. Washing anti-Ig. G-coated indicator red cells are added. centrifuge
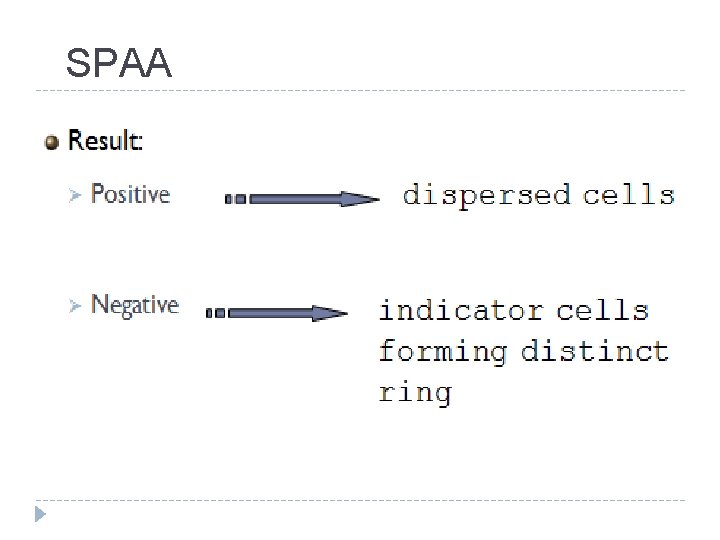
SPAA
Post-analytical phase Involves labeling, inspecting, and issuing the blood unit Labeling form includes patient’s full name, ID number, Location, ABO/Rh(D) of patient and unit, donor #, compatibility results, and tech ID Form is attached to the donor unit and only released for the recipient The unit is visually inspected for abnormalities, such as bacterial contamination, clots, etc

Issuing blood When it’s time to release a blood product to the nurse or physician, a few “checks” must be done Requisition form Comparing requisition form donor unit tag blood product label Name of persons issuing and picking up blood Date and time of release Expiration date

What if the unit is unused? Blood can be returned to the blood bank if it is not needed for transfusion Unit closure has to remain unopened Storage temperature must have remained in the required range (1° to 10°C for RBCs) If not at correct temp, unit must be returned within 30 minutes of issue
Special Circumstances

Emergency Release In an emergency, there may not be enough time to test the recipient’s sample In this case, blood is released only when signed by the physician (O negative) The tag must indicate it is not crossmatched Segments from the released units should be retained for X-matching Every detail is documented (names, dates. . )

Emergency Release Once the specimen is received, ABO/Rh typing and antibody screening should be performed Crossmatching the segments from the released unit should be tested In addition, the lab may crossmatch additional units as a precaution if more blood is needed If death should occur, testing should be complete enough to show that the death was unrelated to an incompatibility

What can be given in an emergency? Group O Rh(D)-negative red cells or AB plasma Emergency release Women below or of childbearing age Group O Rh(D)-positive red cells Used as a substitution if O negative is not available Male or elderly females
Massive transfusion Defined as a transfusion approaching or exceeding the recipient’s own blood volume (about 5 liters or 10 -12 units in an adult male) within 24 hour period The original sample no longer represents the patient’s condition Complete Crossmatch not necessary (if no antibodies were detected originally) Give ABO identical units If antibodies were originally ID’s, continue to give antigen negative units
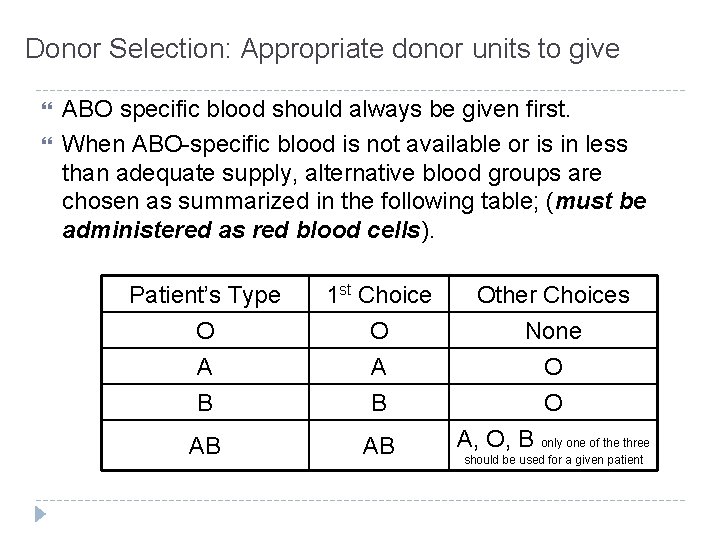
Donor Selection: Appropriate donor units to give ABO specific blood should always be given first. When ABO-specific blood is not available or is in less than adequate supply, alternative blood groups are chosen as summarized in the following table; (must be administered as red blood cells). Patient’s Type O A B 1 st Choice O A B AB AB Other Choices None O O A, O, B only one of the three should be used for a given patient

Selection of Appropriate Donor Units. Rh-negative blood can be given to Rh-positive patients, however, good inventory management should conserve this limited resource for use in Rhneg recipients. If Rh-neg units is near expiration, the unit should be given rather than wasted.
Selection of Appropriate Donor Units. Rh-pos blood should not be given to Rh(D) -neg women of childbearing age. Transfusion of Rh-neg male patients and female patients beyond menopause with Rh-pos blood is acceptable as long as no performed anti-D is demonstrable in the sera.
Major Crossmatch Tests It is done both for Ig. M and Ig. G antibodies Requirement: Recipient’s serum. Donor’s red cells taken from the tube attached to the bag.
A-Saline technique is designed to detect compatibility of Ig. M antibody(ies) in patient’s serum against antigens on donor’s red cells. Method: 1. Label 1 tube for each donor sample to be tested. 2. Put 2 drop of patient’s serum in labeled tube. 3. Add 1 drop of 2 -5% saline suspended red cells of donor 4. Mix and incubate for 5 -10 min. (spin method) or incubate for 30 -60 min (sedimentation method) at RT. 5. Centrifuge at 1000 rpm for 1 min. in spin method (after 5 -10 min. incubation); centrifugation is optional in sedimentation method.

6. Read the result, observe for hemolysis and agglutination. 7. Negative result should be confirmed under microscope. � Interpretation � Agglutination or hemolysis indicates a positive result (incompatible) � Note: In emergency spin technique is acceptable. � Saline technique is inadequate as a complete compatibility test because it is inadequate to detect clinically significant Ig. G antibodies.

B- Anti -Human Globulin Test (IAT) Indirect anti human globulin test (IAT) is the most important and widely used serological procedure in modern blood banking to test the Ig. G compatibility between recipient’s serum and donor’s cells. The majority of incomplete antibodies are Ig. G and are detected by AHG test.

Method 1. Put 2 drops of patient’s serum in a labeled tube. 2. Add 1 drop of 2 -5 % saline suspended red cells of donor. 3. Incubate for 30 -60 min at 37° C 4. Centrifuge at 1000 rpm for 1 min, check for hemolysis/agglutination 5. If there is no hemolysis /agglutination, wash the cells three times with normal saline.

6. Perform IAT test • • • Add 2 drops of polyspecific AHG serum to washed cells Centrifuge at 1000 rpm for 1 minute See for agglutination 7. Add Ig. G coated red cells to negative AHG test. 8. Centrifuge and check for agglutination - if there is no agglutination test is invalid.
Interpretation Hemeolysis or agglutination at any stage indicates incompatibility. Note: Cross-match can be done by two tubes technique for Ig. M and Ig. G separately as described above or by one tubes in which donor’ cell and the patient’s serum after step 5 in saline technique is incubated at 37°C for 20 -30 minutes and then do IAT. In major-cross for Ig. G antibodies albumin or enzyme or LISS can be used with IAT to increase sensitivity. For techniques see chapter on Antiglobulin Test.
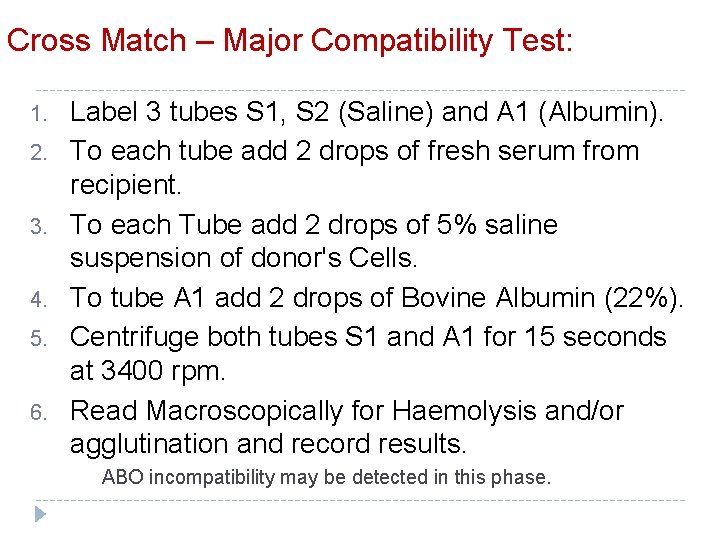
Cross Match – Major Compatibility Test: 1. 2. 3. 4. 5. 6. Label 3 tubes S 1, S 2 (Saline) and A 1 (Albumin). To each tube add 2 drops of fresh serum from recipient. To each Tube add 2 drops of 5% saline suspension of donor's Cells. To tube A 1 add 2 drops of Bovine Albumin (22%). Centrifuge both tubes S 1 and A 1 for 15 seconds at 3400 rpm. Read Macroscopically for Haemolysis and/or agglutination and record results. ABO incompatibility may be detected in this phase.

7. 8. 9. 10. 11. 12. 13. 14. Incubate the Tube S 1 at room temperature for 15 min (Optional). Incubate the Tube S 2 and A 1 in the water bath for 30 min at 37 o C. When the incubation time finished centrifuge the tube/tubes for 15 second at 3400 rpm. Read the tube/tubes macroscopically for Haemolysis and/or agglutination and record results. Wash Tube A 1 with saline 3 times. Add drops of Anti Human Globulin serum and mix well. Centrifuge tube A for 15 second at 3400. Read for agglutination and record the results.
Interpretation: If no agglutination of Haemolysis is present in corssmatch procedure, the blood is regarded compatible and reported as crossmatch Negative.
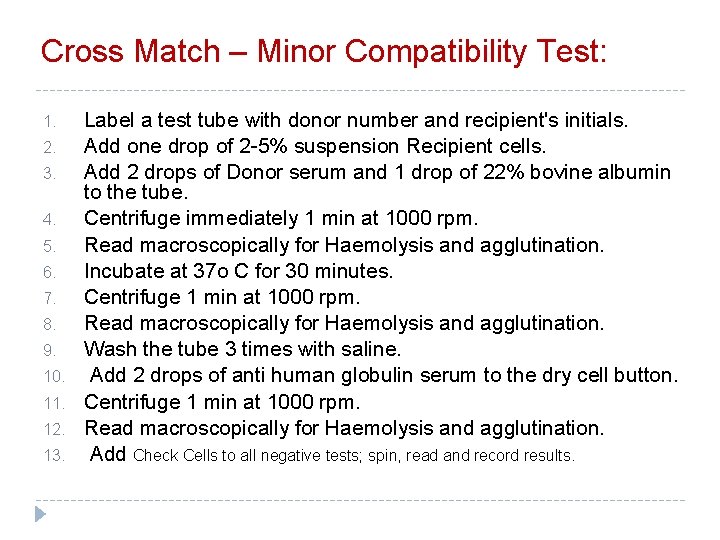
Cross Match – Minor Compatibility Test: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Label a test tube with donor number and recipient's initials. Add one drop of 2 -5% suspension Recipient cells. Add 2 drops of Donor serum and 1 drop of 22% bovine albumin to the tube. Centrifuge immediately 1 min at 1000 rpm. Read macroscopically for Haemolysis and agglutination. Incubate at 37 o C for 30 minutes. Centrifuge 1 min at 1000 rpm. Read macroscopically for Haemolysis and agglutination. Wash the tube 3 times with saline. Add 2 drops of anti human globulin serum to the dry cell button. Centrifuge 1 min at 1000 rpm. Read macroscopically for Haemolysis and agglutination. Add Check Cells to all negative tests; spin, read and record results.

Interpretation: If no agglutination of Haemolysis is present in corssmatch procedure, the blood is regarded as serological compatible and reported as crossmatch Negative.
- Slides: 56