Practical Absorbance and Fluorescence Spectroscopy Chapter 2 Wavelengths

Practical Absorbance and Fluorescence Spectroscopy Chapter 2
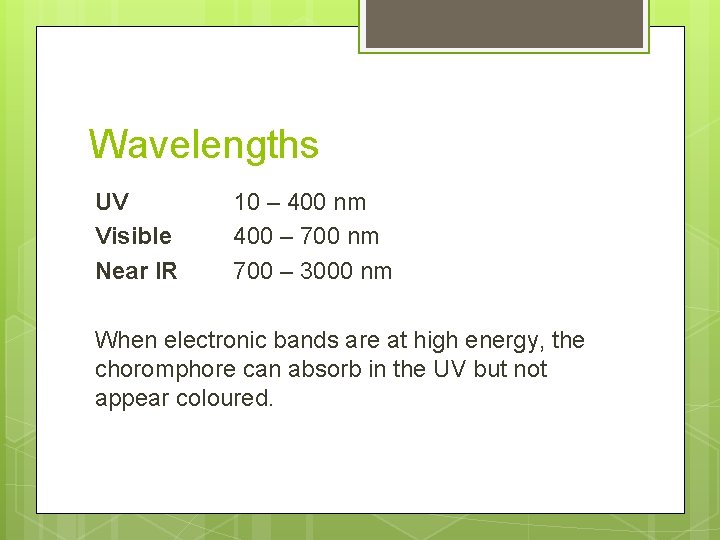
Wavelengths UV Visible Near IR 10 – 400 nm 400 – 700 nm 700 – 3000 nm When electronic bands are at high energy, the choromphore can absorb in the UV but not appear coloured.

Absorption and Fluorescence Absorption A single electron being promoted to a higher energy orbital on absorption of a photon. Fluorescence Absorption whereby the energy is lost by emitting a photon rather than through heat.
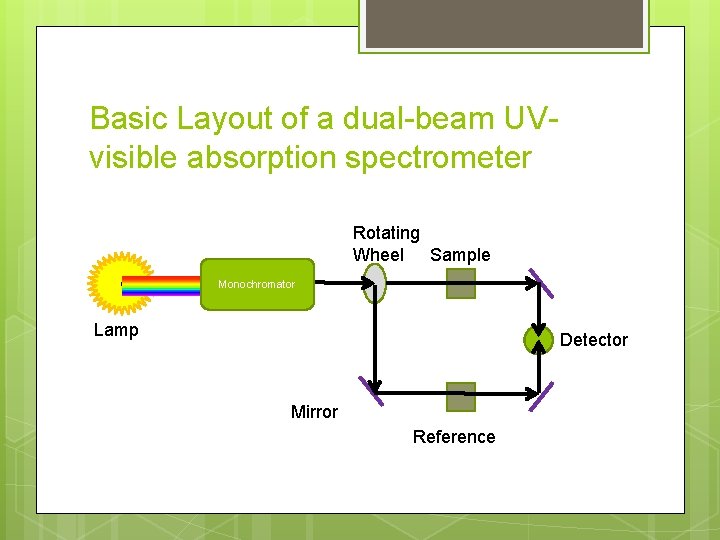
Basic Layout of a dual-beam UVvisible absorption spectrometer Rotating Wheel Sample Monochromator Lamp Detector Mirror Reference

Absorbance and Beer-Lambert Law

Basic Layout of a Fluorimeter PM T Sample Monochromator Lamp Excitation Monochromator Spectrum of Emission Excitation spectrum should look like absorption PM T Emission
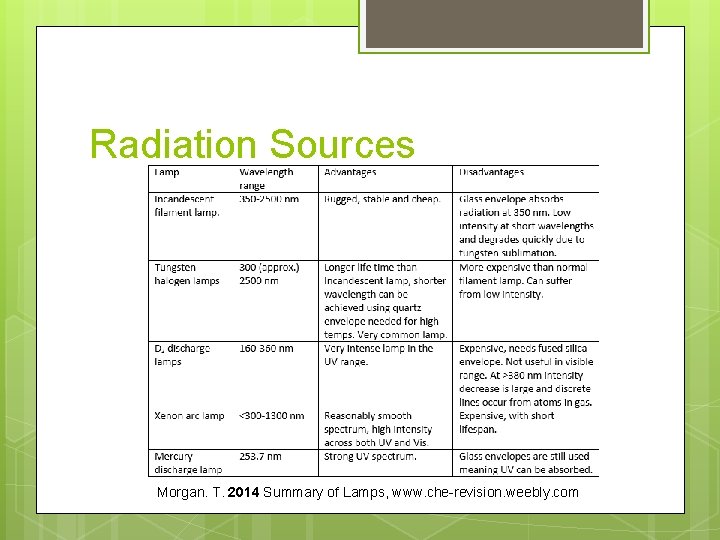
Radiation Sources Morgan. T. 2014 Summary of Lamps, www. che-revision. weebly. com

Wavelength Selection Absorption Filters Combine to select narrow bands of frequencies Interference Filters Relies on optical interference
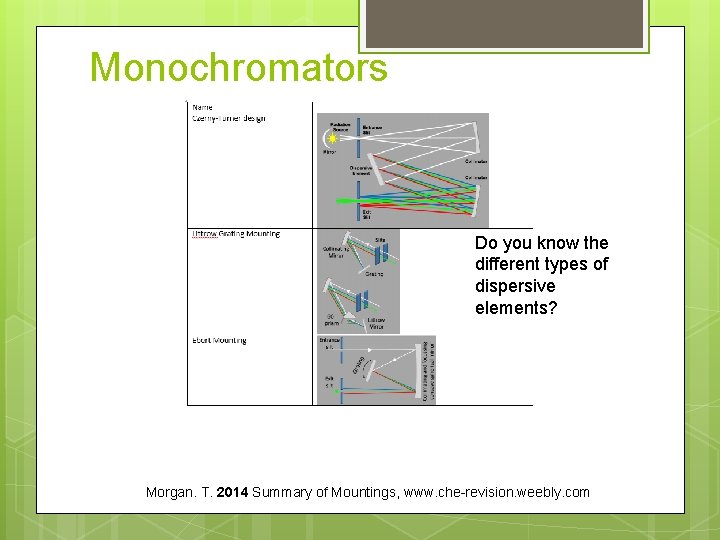
Monochromators Do you know the different types of dispersive elements? Morgan. T. 2014 Summary of Mountings, www. che-revision. weebly. com

Slits (giggedy) Slits Controls luminous flux from monochromator Also controls spectral bandwidth Spectral Bandwidth Monochromator cannot isolate a single wavelength. A definite band is passed. Long narrow slit with adjustable width allowing selection of bandwidth.

Monochromator Performance Resolution Distinguish dispersion adjacent features depends on Purity Amount Light of stray or scattered radiation Gathering Power Improved by power of source, but compromised by narrower slit to maintain resolution

Monochromator Performance

Dispersion
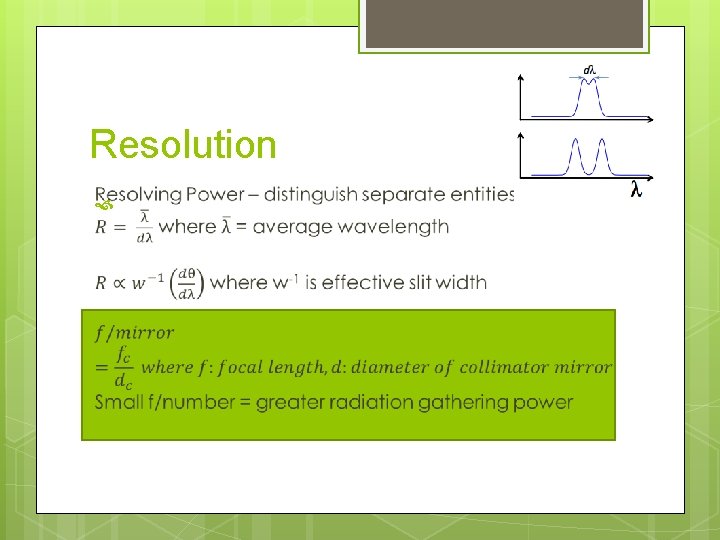
Resolution
Detectors Transducers that converts electromagnetic radiation into electron flow Uses Photoelectric Effect E = hv – w (w = work function) Need to know the different types of detectors

Fluorescence in Detail Excited electronic state Fluorescence only occur from v = 0 state of S 1 to any sub-level of S 0 Ground electronic state
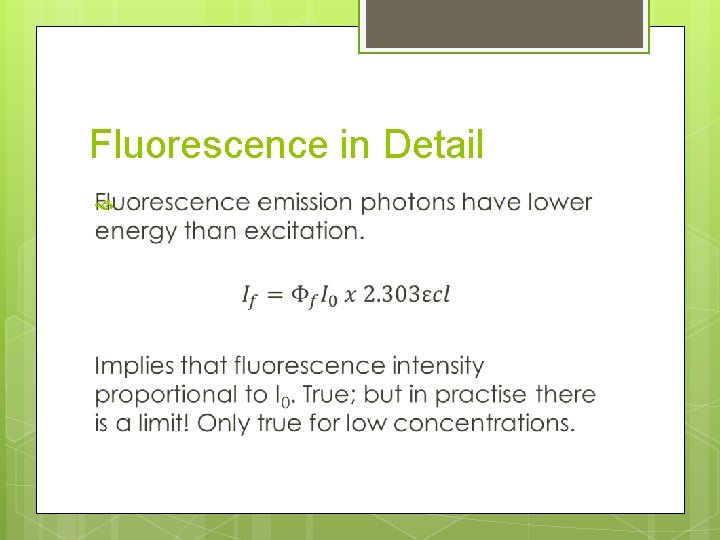
Fluorescence in Detail
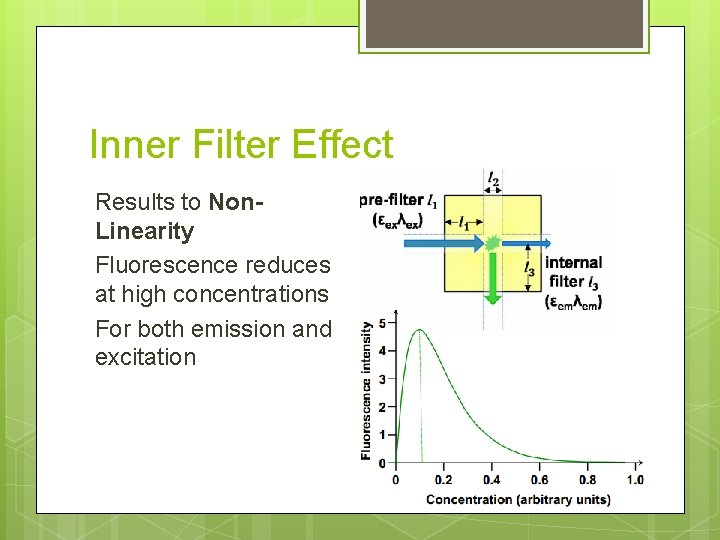
Inner Filter Effect Results to Non. Linearity Fluorescence reduces at high concentrations For both emission and excitation
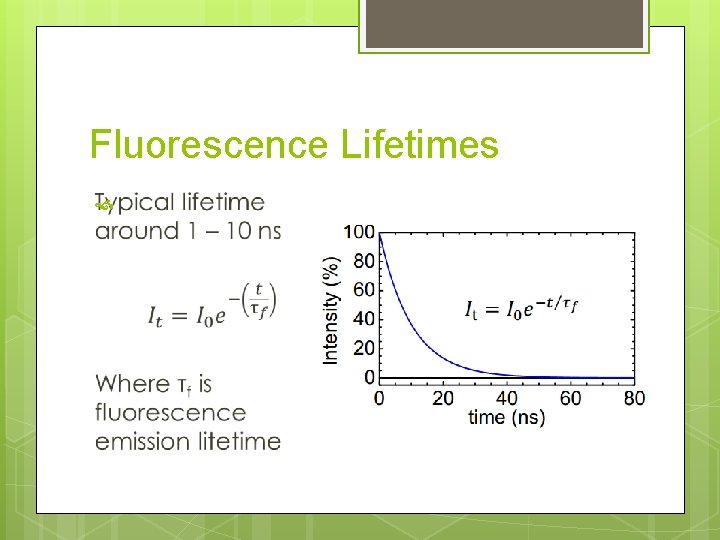
Fluorescence Lifetimes
Fluorescence Quantum Yields Φf = fluorescence quantum yield Fraction of excited state molecules that decay back to ground state via fluorescence photons Between 0 – 1 Polar environments reduce Φf Φf also very dependent on ionisation (switch from fluo to non-fluo etc…)
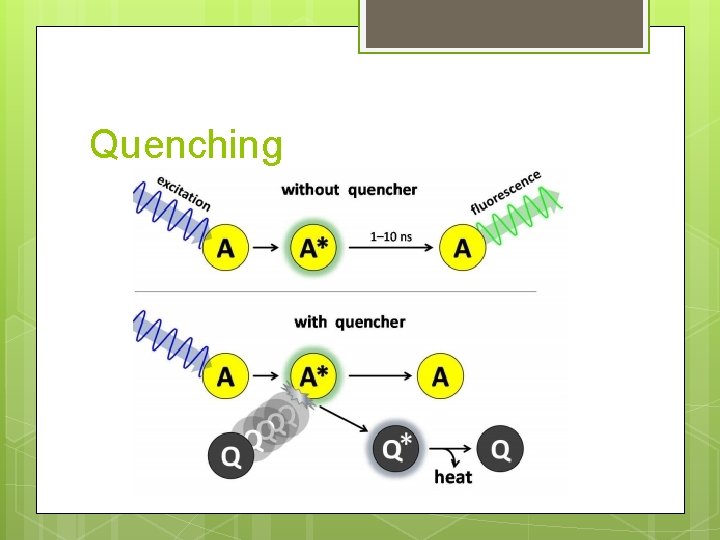
Quenching
Cuvettes EDC Quartz Optical Glass ES Quartz IR Quartz 200 – 2800 nm 300 – 2600 nm 190 – 2000 nm 300 – 3500 nm Therefore for UV <300 nm, need quartz not glass. Plastic can be used in visible (polystyrene is fluorescent; PMMA ‘poly(metyl methacrylate)’ used instead)
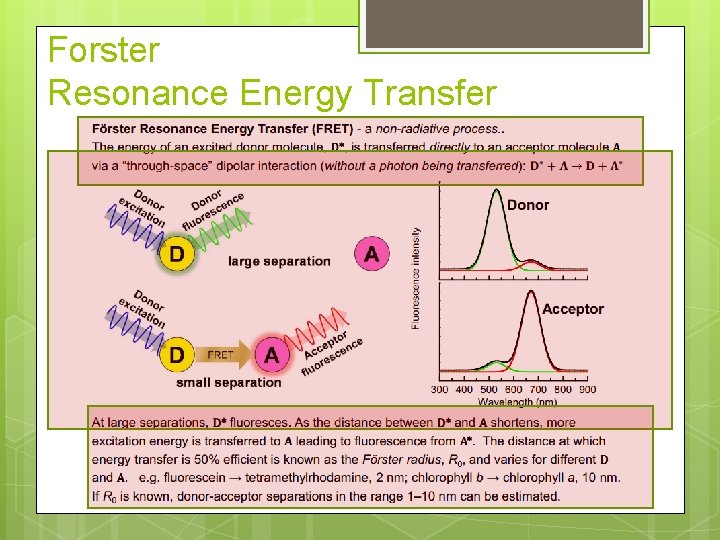
Forster Resonance Energy Transfer
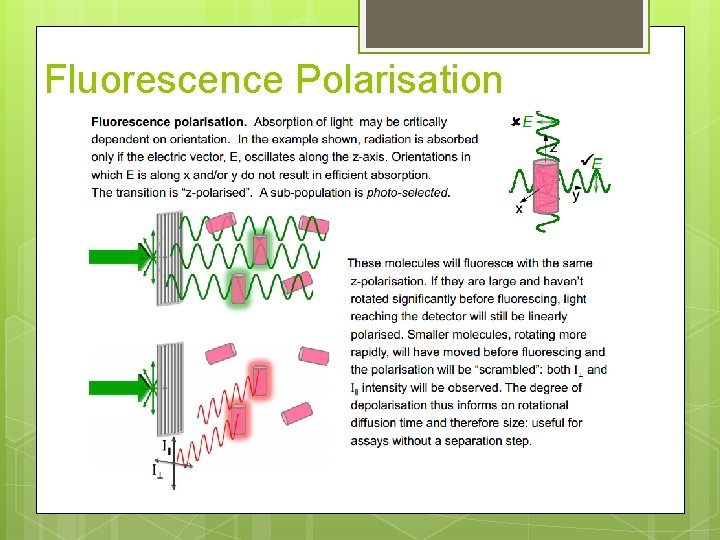
Fluorescence Polarisation
- Slides: 24