PPT 2019 Ionic Liquid ILs Dr MAHMOUD Najim






![Typical Cation and Anion Typical IL Cations * [PF 6]- for moisture stable, water Typical Cation and Anion Typical IL Cations * [PF 6]- for moisture stable, water](https://slidetodoc.com/presentation_image_h/2cbb2529a4886bf23ccb5e5f31a70b1e/image-14.jpg)



- Slides: 28

PPT 2019 Ionic Liquid ILs Dr MAHMOUD Najim Abid & Taghreed M. Musa

One of the main directions of the global research , is the search for new chemical compounds with special , properties. Ionic liquids are such compounds Their application brings new , possibilities for modern chemical technology. The ionic liquids, fit, well, in the assumptions of green chemistry. In contrast to the previous approach , , the green chemistry requires design, , development and implementation of new processes and chemicals that allow the reduction or elimination of use and production of hazardous, materials. Twelve principles of green chemistry formulated in 1998 by Anastas and Warner describe the methods of implementation of these tasks. Ionic liquids meet at least three of these principles (safer solvents) (provide energy efficiency) and no. 9 (are used in catalytic reactions). Ionic liquid precursors are quaternary ammonium halides known from the 1890 s , which were widely used and tested in the 20 th century. The history of synthesis and application of organic salts containing a quaternary nitrogen atom is presented

Discovery and History * The description of a low melting point salt 1914 Ethyl ammonium nitrate * The first room temperature ionic liquid -1951 N-ethylpyridinium bromide- aluminium chloride melt * The most stable and conductive salts 1982 1, 3 -dialkylimidazolium salts * The hydrophobic ionic liquids 1992 1 -ethyl-3 -methylimidazolium tetrafluoroborate

Several experiments have conducted to prepare ionic liquids used different types of Amines components as Ethanolamine, Urea, Acetamide, Thiourea and Thioacetmide, with aluminum salt as Aluminum Chloride, Aluminum nitrate, Ammonium Sulfate, Potassium Aluminum Sulfate and Aluminum Stearate. Some of these attempts have produced results using varying temperatures of up to 80°C and other auxiliary factors. Some physical characteristics such as ionic conductivity and melting point. FT-IR and electronic spectra and cyclic voltametry measurements have investigated

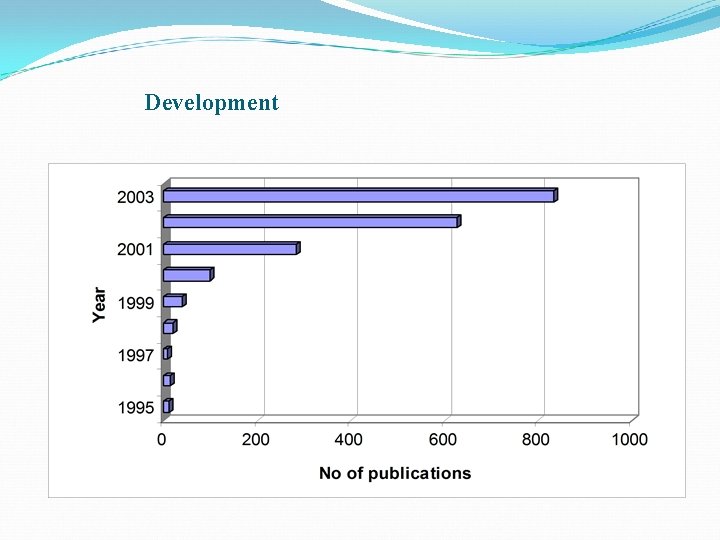
Development

Solvents – classification Organic solvents involve Vander Walls interaction Water Hydrogen bonding Ionic Liquids involves columbic interactions between assymetric cation And symmetric anions like BF 4 - , PF 6 -, Cl. O 4 - The asymmetry of cations in the structure of Ils will prevent the packing Of ions and no lattice as shown in ionic compounds and this unique Properties makes the Ils have extreme physical and chemical properties And then it is called future and green solvents

What are Ionic Liquids Ionic liquid in synthesis

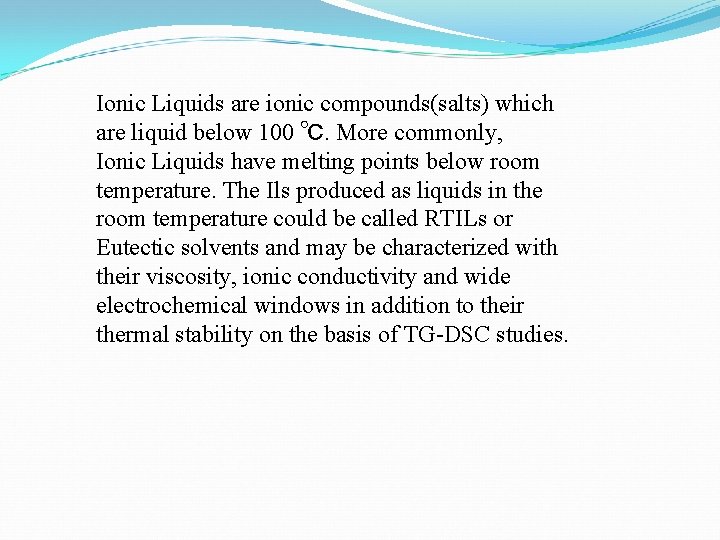
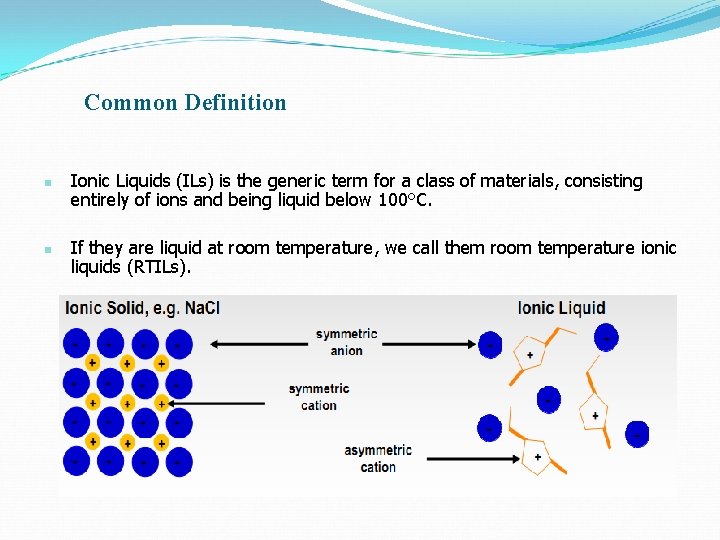
Ionic Liquids are ionic compounds(salts) which are liquid below 100 ℃. More commonly, Ionic Liquids have melting points below room temperature. The Ils produced as liquids in the room temperature could be called RTILs or Eutectic solvents and may be characterized with their viscosity, ionic conductivity and wide electrochemical windows in addition to their thermal stability on the basis of TG-DSC studies.

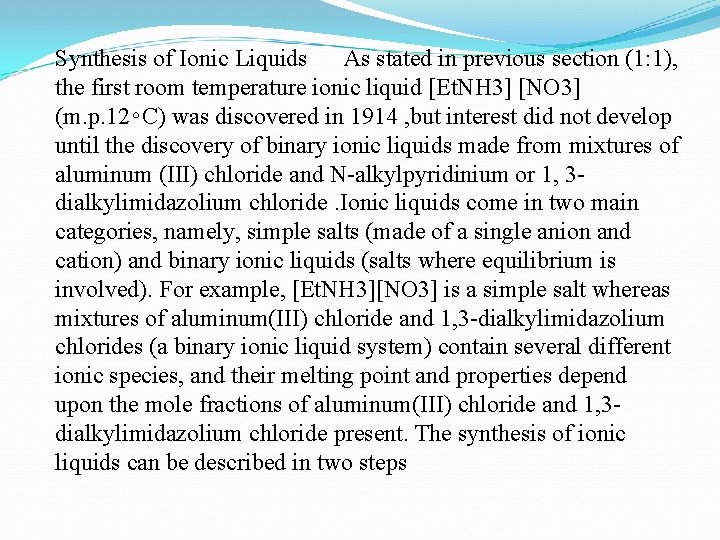
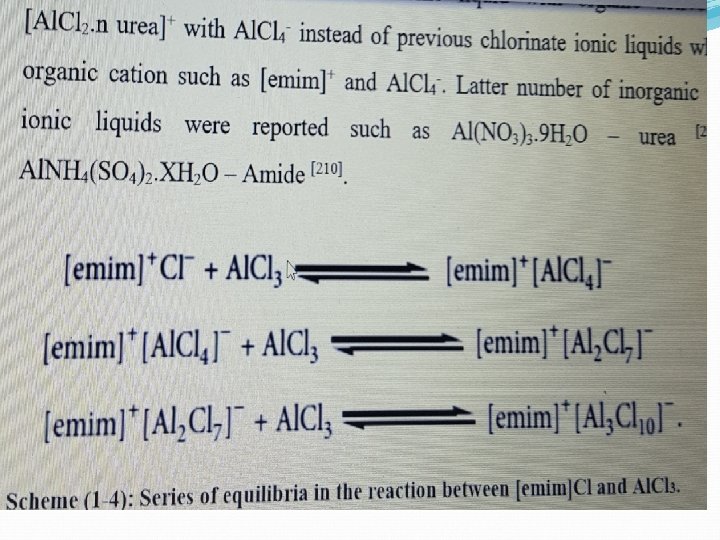
Synthesis of Ionic Liquids As stated in previous section (1: 1), the first room temperature ionic liquid [Et. NH 3] [NO 3] (m. p. 12∘C) was discovered in 1914 , but interest did not develop until the discovery of binary ionic liquids made from mixtures of aluminum (III) chloride and N-alkylpyridinium or 1, 3 dialkylimidazolium chloride. Ionic liquids come in two main categories, namely, simple salts (made of a single anion and cation) and binary ionic liquids (salts where equilibrium is involved). For example, [Et. NH 3][NO 3] is a simple salt whereas mixtures of aluminum(III) chloride and 1, 3 -dialkylimidazolium chlorides (a binary ionic liquid system) contain several different ionic species, and their melting point and properties depend upon the mole fractions of aluminum(III) chloride and 1, 3 dialkylimidazolium chloride present. The synthesis of ionic liquids can be described in two steps

Main routes to prepare Ionic Liquids: 1 -Acid base reactions like thiourea with salts of Calcium or alumium 2 -Solvation of active organic compounds like citrazine, triacetic acid with some metal salts 3 -Metathesis reactions which involves the displacement reactions 4 -Alkylation of heterocyclic compounds like pyridine, pyrrolidine and imidazole with R-X followed metathesis reaction by salts of KPF 6, KBF 4 and metal perchlorate or bromate 5 -Rare reactions of chelation which are almost occurred in the case of 18 -crown-6 with K+ and Li+ ions then fast metathesis with symmetric anions of PF 6 -, CF 3 COO- and BF 4 -.


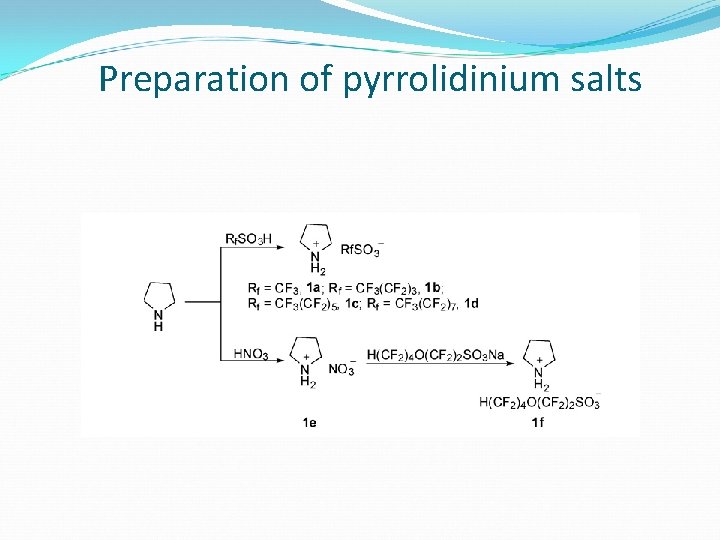
Preparation of pyrrolidinium salts

Common Definition n n Ionic Liquids (ILs) is the generic term for a class of materials, consisting entirely of ions and being liquid below 100°C. If they are liquid at room temperature, we call them room temperature ionic liquids (RTILs).
![Typical Cation and Anion Typical IL Cations PF 6 for moisture stable water Typical Cation and Anion Typical IL Cations * [PF 6]- for moisture stable, water](https://slidetodoc.com/presentation_image_h/2cbb2529a4886bf23ccb5e5f31a70b1e/image-14.jpg)
Typical Cation and Anion Typical IL Cations * [PF 6]- for moisture stable, water immiscible IL * [BF 4]- for moisture stable, but water miscible IL * [Al. Cl 4 -] (or other Lewis acids) decomposes in water

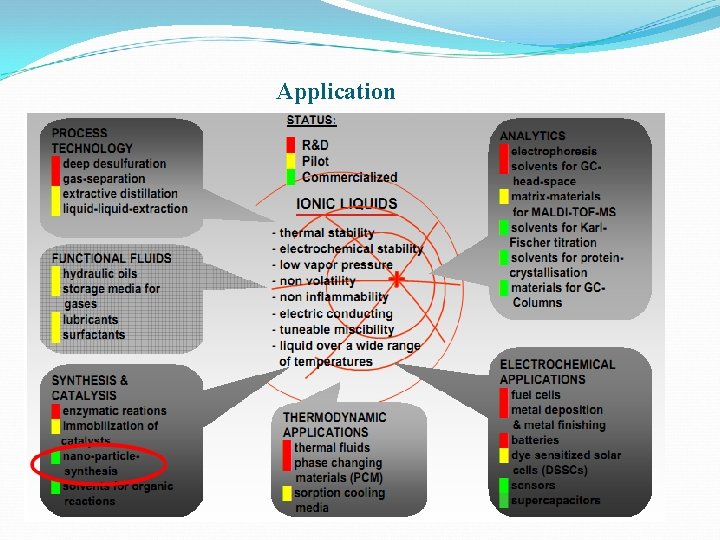
Application

Advantage Easy separation Very low vapor pressure Non-flammable substance High thermally stable High mechanically stable Electrochemically stable Low toxicity Non-volatility

Ionic Liquids in Organic Synthesis Catalytic Friedel–Crafts Hydrogenetions Alkoxycarbonylation Hydroformylations Heck reactions Olefin dimerization Diels-Alder Suzuki coupling Stille Coupling Oxidations

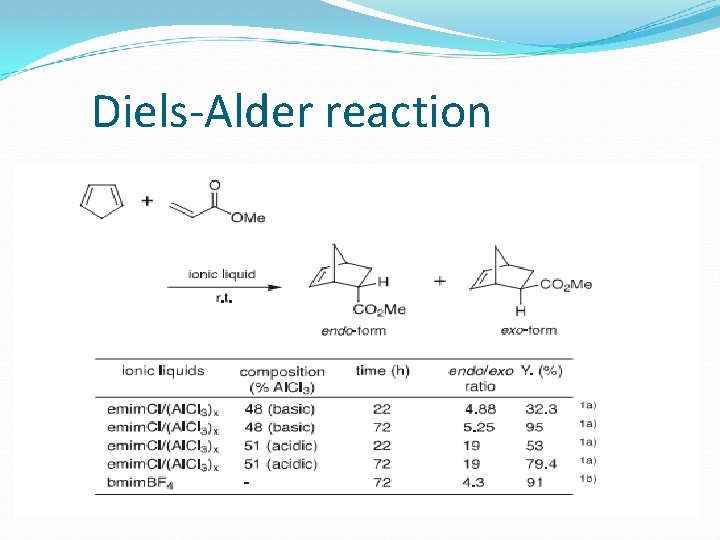
Diels-Alder reaction

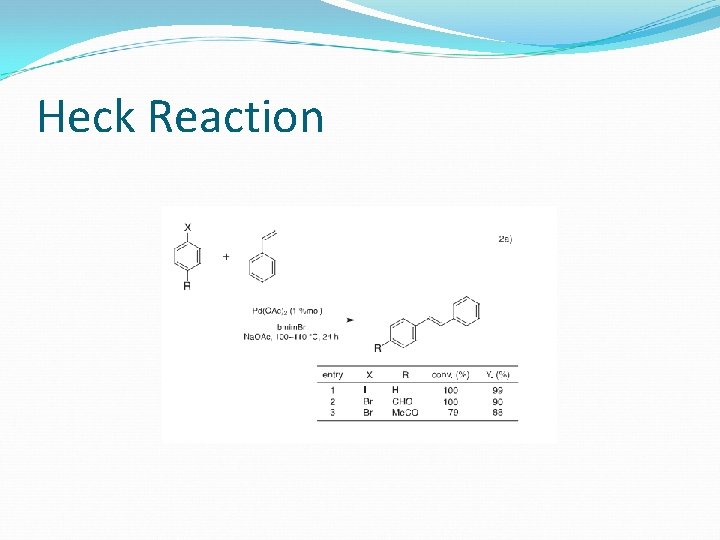
Heck Reaction

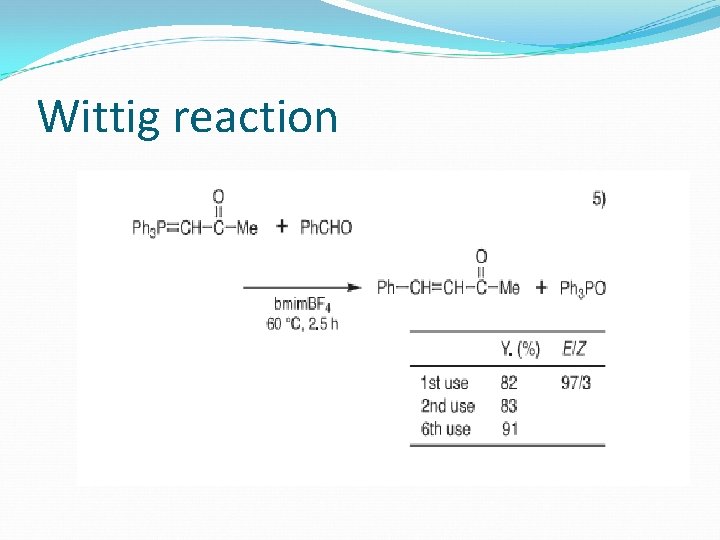
Wittig reaction

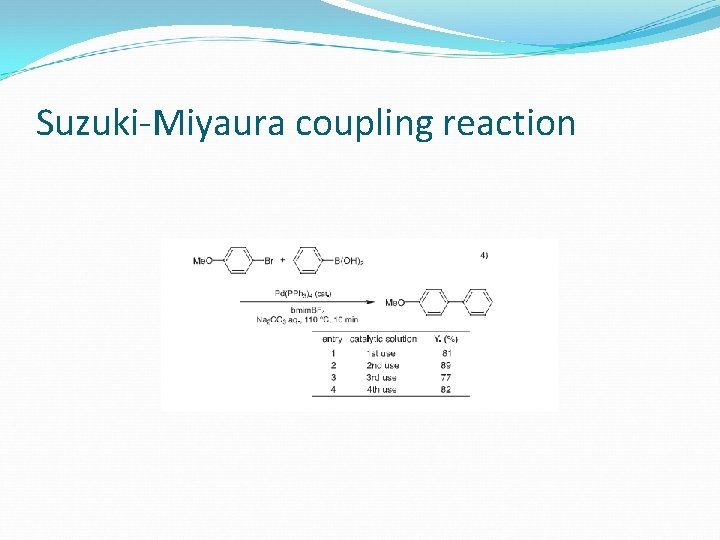
Suzuki-Miyaura coupling reaction

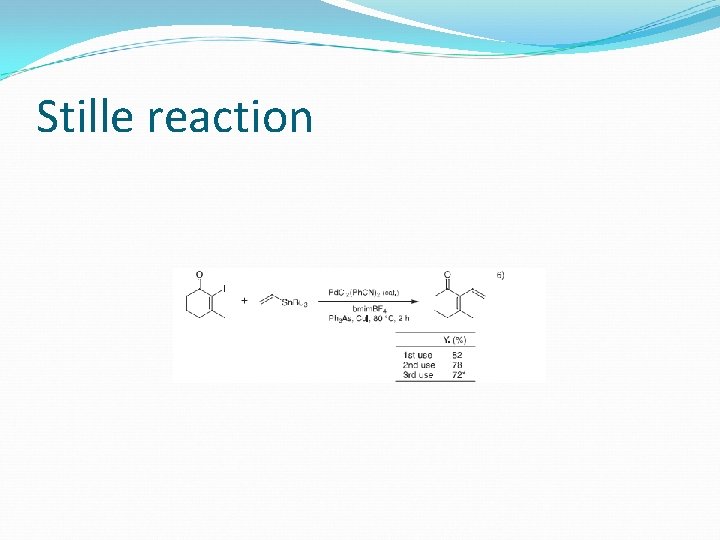
Stille reaction

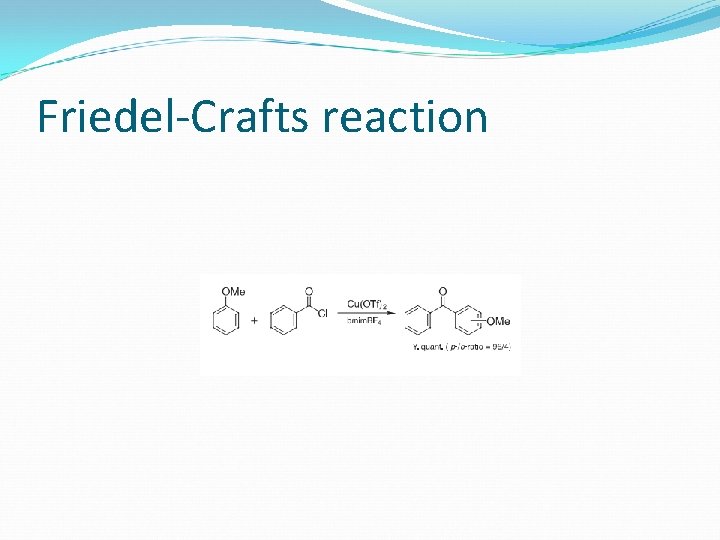
Friedel-Crafts reaction

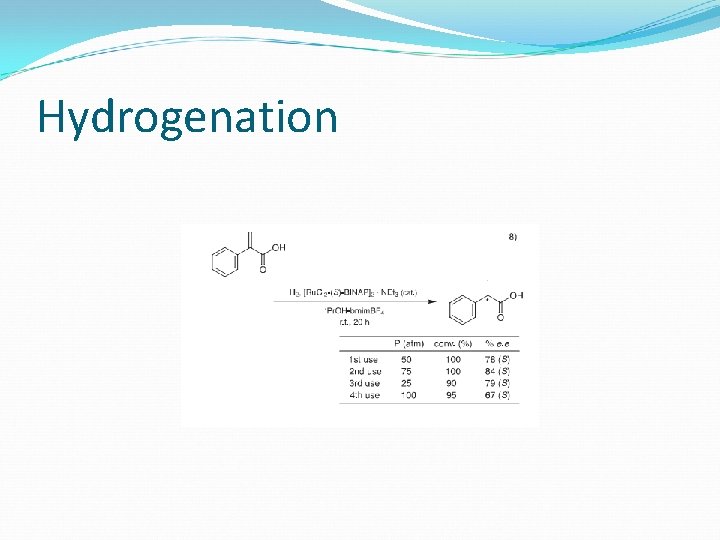
Hydrogenation

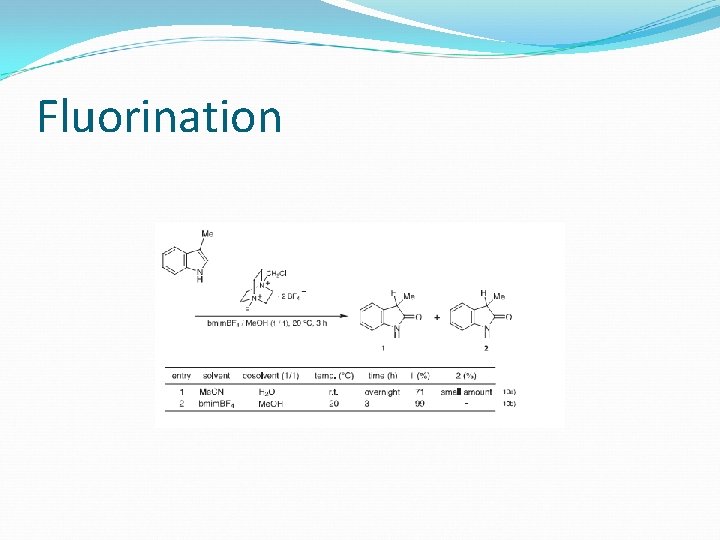
Fluorination

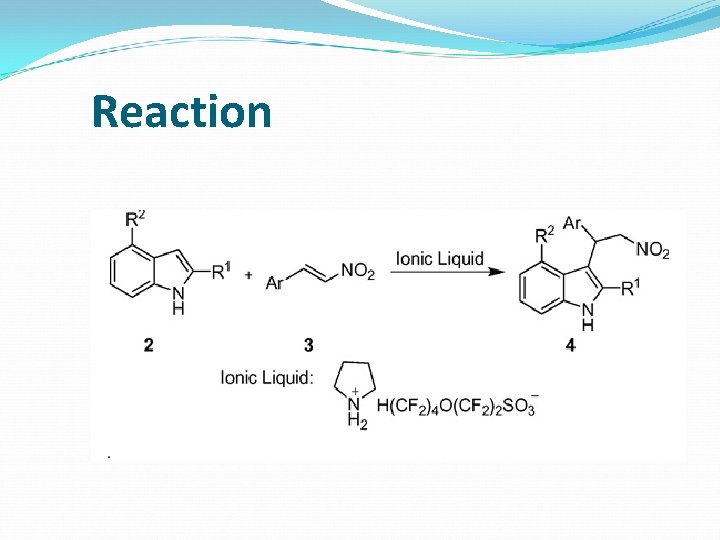
Reaction

We can in the future research 1. Prepare a new ionic liquids by mixing the other aluminum M = Zn, Sn, Fe, Al, Ga, Ca, salts with organic compound. 2. Study the status stereochemistry and geometric shape of ionic liquids prepared 3. Study the possibility of using ionic liquids as an antibiotic or with other pharmaceuticals. 4. The possibility of studying the effect of ionic fluids on hormones and cancer tumors 5. The toxic influence of ILs may be associated to a common cellular structure or process

Conclusion Ionic liquids , as the new materials of multifunction , are widely used in various of fields. Environmently-friendly reaction process have vigorously been studied from the standpoint of green chemistry and based on the properities of easy separation , low toxicity , selective miscibility , ILS play an important role in organic synthesis as the green alternative solvent. Withe development of multifunctional ILS , we can expect Il. S would apply in more fields.