Ppt 16 a OxidationReduction Reactions 1 Overview Importance








- Slides: 17

Ppt 16 a, Oxidation-Reduction Reactions 1. Overview, Importance 2. Definitions, Language Issues – Oxidation and “variants”: to get oxidized; to oxidize (somebody); oxidizing agent – Reduction and “variants”: to get reduced; to reduce (somebody); reducing agent 3. Concept of Oxidation Numbers (States) – For when species are not monatomic 4. How to Assign Oxidation Numbers 5. How to Use Oxidation Numbers to Assess Redox and determine the oxidizing agent and/or reducing agent Ppt 16 a 1

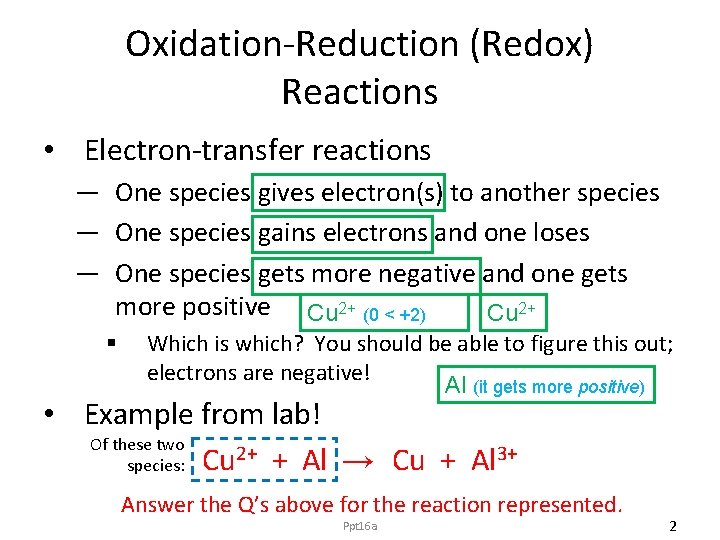
Oxidation-Reduction (Redox) Reactions • Electron-transfer reactions — One species gives electron(s) to another species — One species gains electrons and one loses — One species gets more negative and one gets more positive Cu 2+ (0 < +2) Cu 2+ § Which is which? You should be able to figure this out; electrons are negative! Al (it gets more positive) • Example from lab! Of these two 2+ + Al → Cu + Al 3+ species: Cu Answer the Q’s above for the reaction represented. Ppt 16 a 2

Importance? • Redox reactions involve movement of electrons. – Movement of electrons = electric current! – Redox reactions are what “drive” current in batteries! • Makes our Smart. Phones, mp 3 players, etc. work! • As a battery dies, reactants are getting used up and products are forming. • Recharging a battery is making the reverse reaction occur! – No other kind of chemical reaction can be used to make a battery. Very important practically! Ppt 16 a 3

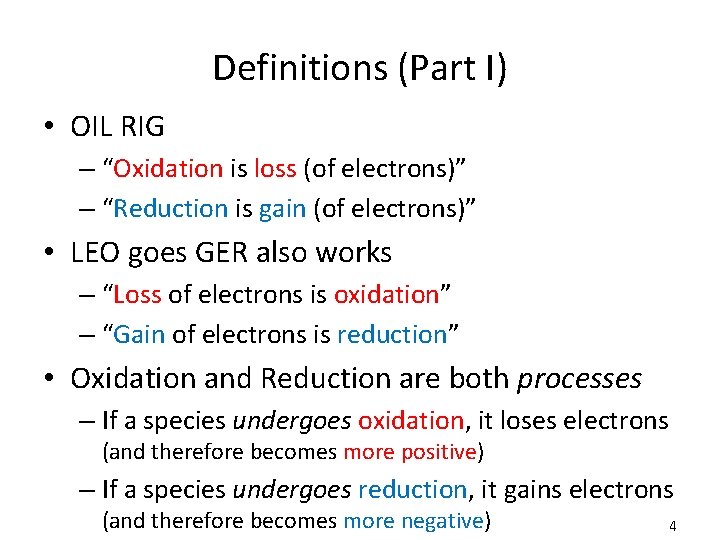
Definitions (Part I) • OIL RIG – “Oxidation is loss (of electrons)” – “Reduction is gain (of electrons)” • LEO goes GER also works – “Loss of electrons is oxidation” – “Gain of electrons is reduction” • Oxidation and Reduction are both processes – If a species undergoes oxidation, it loses electrons (and therefore becomes more positive) – If a species undergoes reduction, it gains electrons (and therefore becomes more negative) 4

Back to Example Cu 2+ + Al → Cu + Al 3+ • Which species underwent reduction? (i. e. , which one gained electrons? ) Ans. Cu 2+; it got more negative. • Which species underwent oxidation? (i. e. , which one lost electrons? ) Ans. Al; it got more positive. Ppt 16 a 5

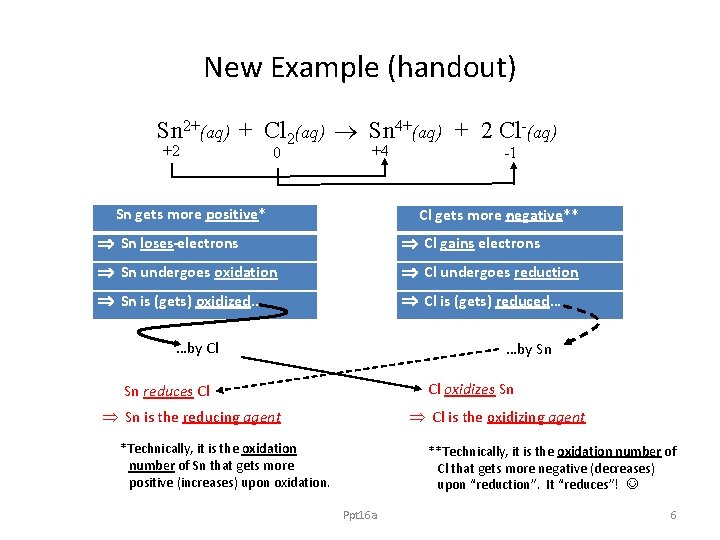
New Example (handout) Sn 2+(aq) + Cl 2(aq) Sn 4+(aq) + 2 Cl-(aq) +2 0 +4 Sn gets more positive* -1 Cl gets more negative** Sn loses electrons Cl gains electrons Sn undergoes oxidation Cl undergoes reduction Sn is (gets) oxidized… Cl is (gets) reduced… …by Cl …by Sn Cl oxidizes Sn Sn reduces Cl Sn is the reducing agent Cl is the oxidizing agent *Technically, it is the oxidation number of Sn that gets more positive (increases) upon oxidation. **Technically, it is the oxidation number of Cl that gets more negative (decreases) upon “reduction”. It “reduces”! Ppt 16 a 6

Oxidation-Reduction Reactions— Language Issues (going a bit farther) 1 a) To reduce (something) means to “give electron(s) to (it)” b/c that will reduce its charge 1 b) A reducing agent is a species that reduces something else. “agent”: person or thing that does something (“acts”) 1 c) If A gets reduced (by B), then A has electrons given to it (by B) 1 d) If A undergoes reduction (by B), then A has electrons given to it (by B) Ppt 16 a 7

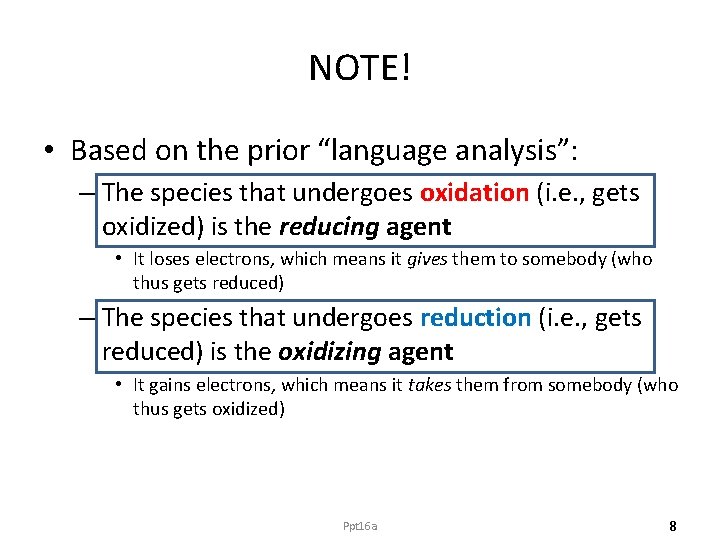
NOTE! • Based on the prior “language analysis”: – The species that undergoes oxidation (i. e. , gets oxidized) is the reducing agent • It loses electrons, which means it gives them to somebody (who thus gets reduced) – The species that undergoes reduction (i. e. , gets reduced) is the oxidizing agent • It gains electrons, which means it takes them from somebody (who thus gets oxidized) Ppt 16 a 8

One last time (all together) Cu 2+ + Al → Cu + Al 3+ Who got more positive (in going from being a R to a P) ? Did that species lose (give) or gain (take) electrons? Did that species reduce or oxidize somebody? Is that species the reducing agent or the oxidizing agent? Did that species undergo reduction or oxidation? Ppt 16 a 9

Oxidation Numbers (States) and application • If all redox reactions involved monatomic species, identification of redox reactions (and ox agent and red agent) would be fairly straightforward. – Figure out who got more positive and who more negative and do as in the prior example • But many redox reactions involve molecules and/or polyatomic ions: CH 4 + O 2 CO 2 + H 2 O [this is redox!] • How to tell? Use oxidation numbers (states) instead of actual charge 10

Oxidation Numbers are assigned to EACH ATOM • Each atom now gets its own “number” • Look to see which atom type (not overall species) gets more positive or negative (look at oxidation number) • If an atom in a species gets more positive, we say that species undergoes oxidation (and is the reducing agent) – Vice versa for the oxidizing agent CH 4 + O 2 CO 2 + H 2 O (on board) Ppt 16 a 11

How to Assign Oxidation Numbers (from handout) Ppt 16 a 12

How to Assign Oxidation Numbers (from handout, continued) Ppt 16 a 13

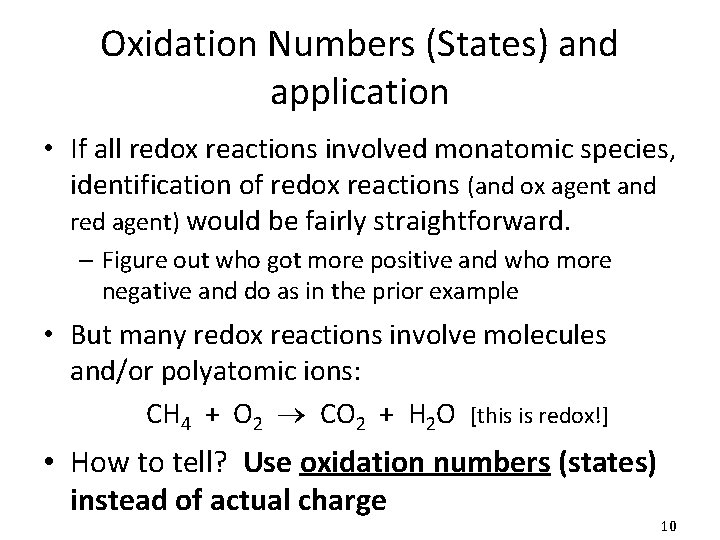
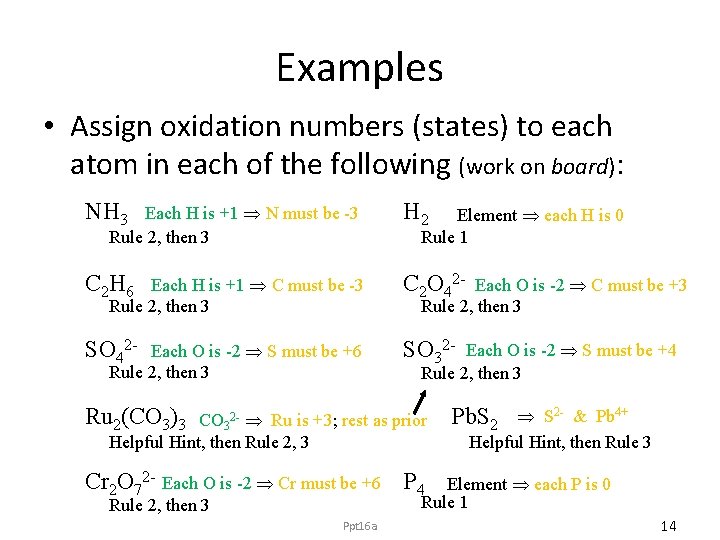
Examples • Assign oxidation numbers (states) to each atom in each of the following (work on board): NH 3 H 2 C 2 H 6 C 2 O 42 - SO 32 - Each H is +1 N must be -3 Rule 2, then 3 Each H is +1 C must be -3 Rule 2, then 3 Each O is -2 S must be +6 Rule 2, then 3 Element each H is 0 Rule 1 Each O is -2 C must be +3 Rule 2, then 3 Each O is -2 S must be +4 Rule 2, then 3 Ru 2(CO 3)3 CO 32 - Ru is +3; rest as prior Helpful Hint, then Rule 2, 3 Pb. S 2 - & Pb 4+ Helpful Hint, then Rule 3 Cr 2 O 72 - Each O is -2 Cr must be +6 P 4 Element each P is 0 Rule 1 Rule 2, then 3 Ppt 16 a 14

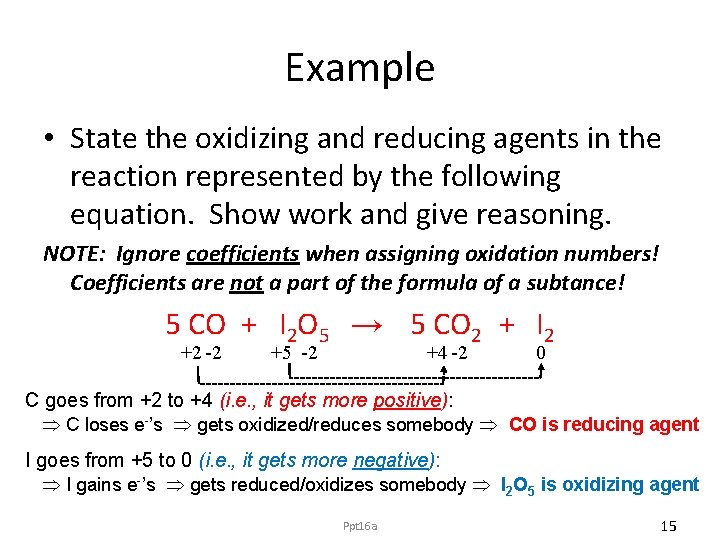
Example • State the oxidizing and reducing agents in the reaction represented by the following equation. Show work and give reasoning. NOTE: Ignore coefficients when assigning oxidation numbers! Coefficients are not a part of the formula of a subtance! 5 CO + I 2 O 5 → 5 CO 2 + I 2 +2 -2 +5 -2 +4 -2 0 C goes from +2 to +4 (i. e. , it gets more positive): C loses e-’s gets oxidized/reduces somebody CO is reducing agent I goes from +5 to 0 (i. e. , it gets more negative): I gains e-’s gets reduced/oxidizes somebody I 2 O 5 is oxidizing agent Ppt 16 a 15

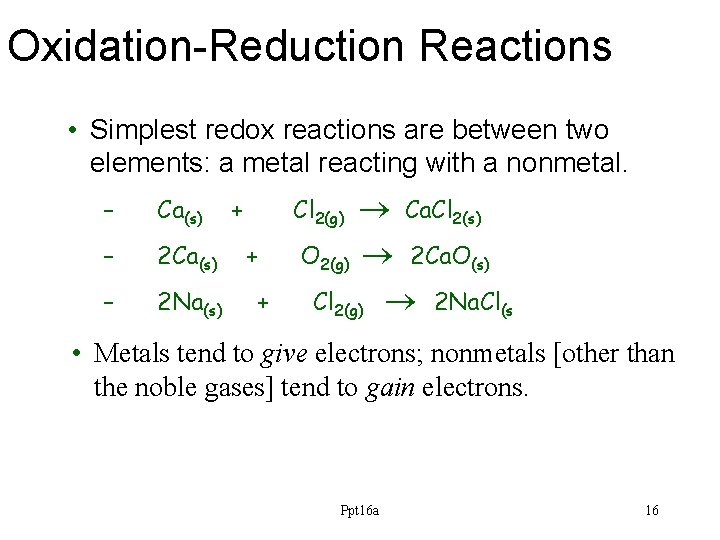
Oxidation-Reduction Reactions • Simplest redox reactions are between two elements: a metal reacting with a nonmetal. – Ca(s) – 2 Na(s) + Ca. Cl 2(s) O 2(g) 2 Ca. O(s) Cl 2(g) 2 Na. Cl(s Cl 2(g) + + • Metals tend to give electrons; nonmetals [other than the noble gases] tend to gain electrons. Ppt 16 a 16

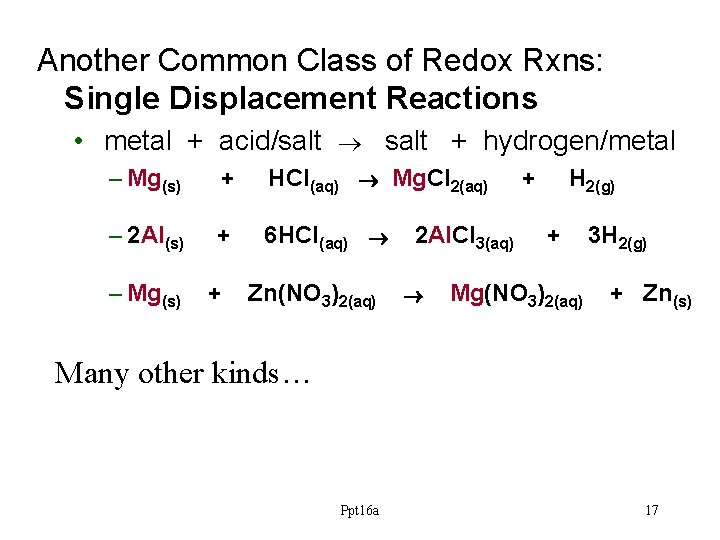
Another Common Class of Redox Rxns: Single Displacement Reactions • metal + acid/salt + hydrogen/metal – Mg(s) + HCl(aq) ® Mg. Cl 2(aq) – 2 Al(s) + 6 HCl(aq) ® – Mg(s) + Zn(NO 3)2(aq) 2 Al. Cl 3(aq) ® + H 2(g) + Mg(NO 3)2(aq) 3 H 2(g) + Zn(s) Many other kinds… Ppt 16 a 17