PP Chames G Rosas L Rem R Willemsen


- Slides: 22

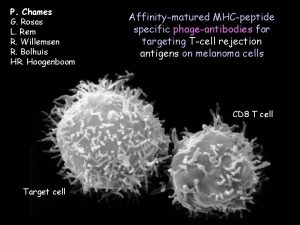
PP. Chames G. Rosas L. Rem R. Willemsen R. Bolhuis HR. Hoogenboom Affinity-matured MHC-peptide specific phage-antibodies for targeting T-cell rejection antigens on melanoma cells CD 8 T cell Target cell

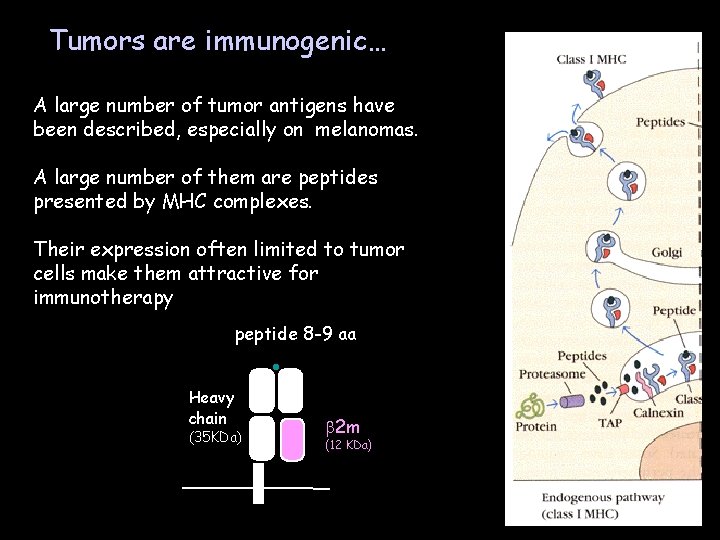
Tumors are immunogenic… A large number of tumor antigens have been described, especially on melanomas. A large number of them are peptides presented by MHC complexes. Their expression often limited to tumor cells make them attractive for immunotherapy peptide 8 -9 aa Heavy chain (35 KDa) b 2 m (12 KDa)

The complex HLA-A 1/MAGE-A 1 • MAGE-A 1 expressed in 40% of melanoma tumors and in some other tumors • 25 % of the population is HLA-A 1 positive => the HLA A 1 -MAGE-A 1 complex is expressed in 10% of all melanoma tumors. • Not expressed at all in normal tissues (apart from testis, but no HLA expression) An antibody directed against this complex would be interesting to: • Check the availability of the complex before immunotherapy (in vitro MAGE-A 1 loaded DC) • As a targeting reagent (immunotoxin/cytokine, T cell retargeting)

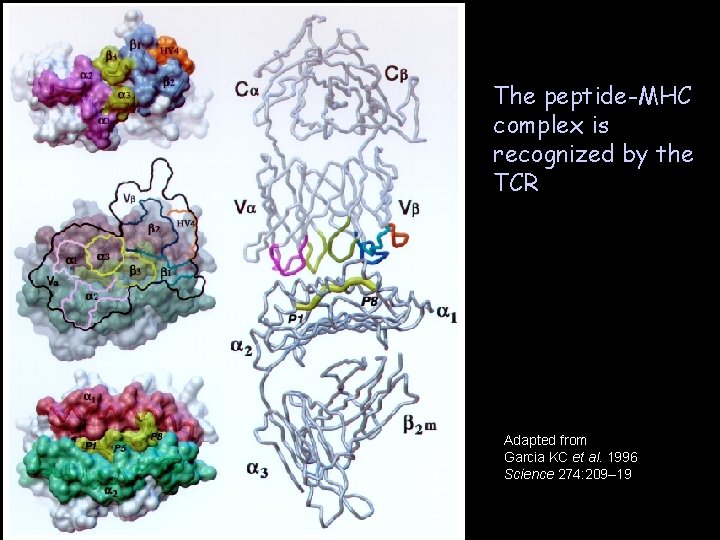
The peptide-MHC complex is recognized by the TCR Adapted from Garcia KC et al. 1996 Science 274: 209– 19

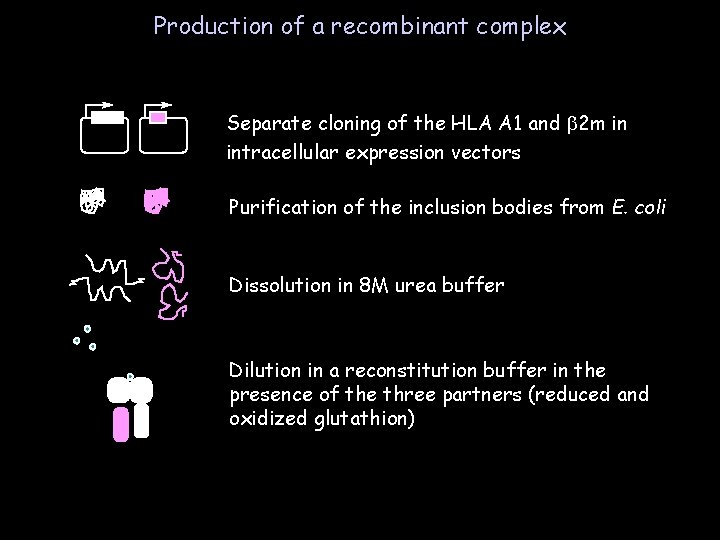
Production of a recombinant complex Separate cloning of the HLA A 1 and b 2 m in intracellular expression vectors Purification of the inclusion bodies from E. coli Dissolution in 8 M urea buffer Dilution in a reconstitution buffer in the presence of the three partners (reduced and oxidized glutathion)

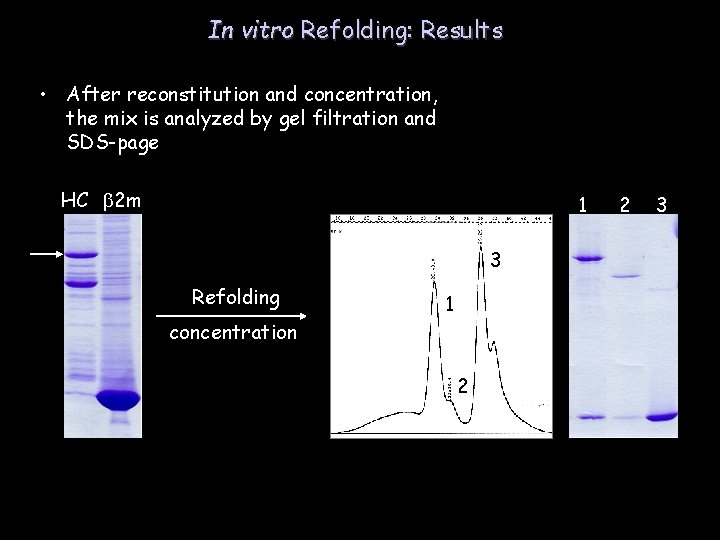
In vitro Refolding: Results • After reconstitution and concentration, the mix is analyzed by gel filtration and SDS-page HC b 2 m 1 2 3 3 Refolding 1 concentration 2 buffer

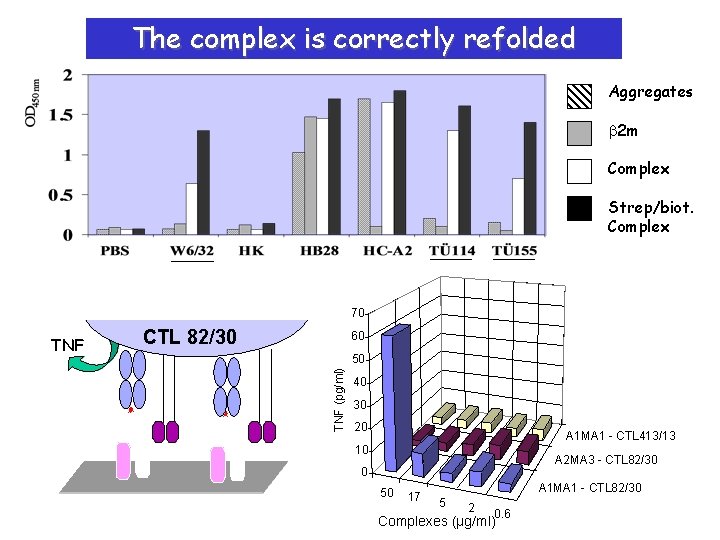
The complex is correctly refolded Aggregates b 2 m Complex Strep/biot. Complex 70 CTL 82/30 60 50 TNF (pg/ml) TNF 40 30 20 A 1 MA 1 - CTL 413/13 10 A 2 MA 3 - CTL 82/30 0 50 17 A 1 MA 1 - CTL 82/30 5 2 0. 6 Complexes (µg/ml)

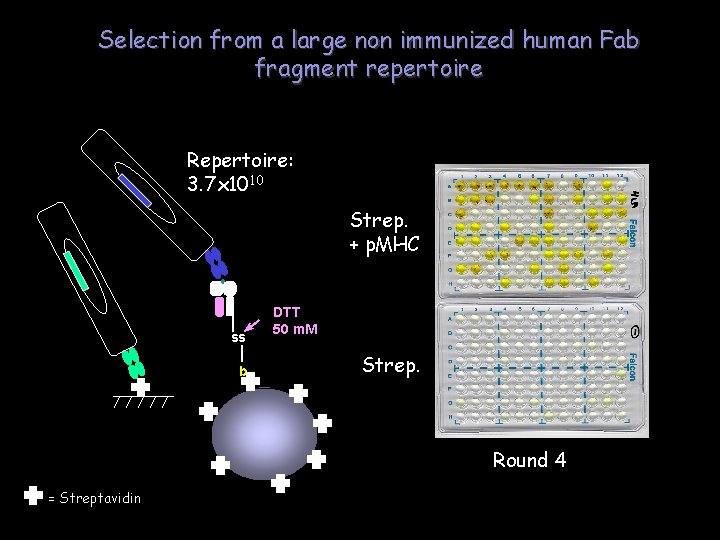
Selection from a large non immunized human Fab fragment repertoire Repertoire: 3. 7 x 1010 Strep. + p. MHC ss b DTT 50 m. M Strep. Round 4 = Streptavidin

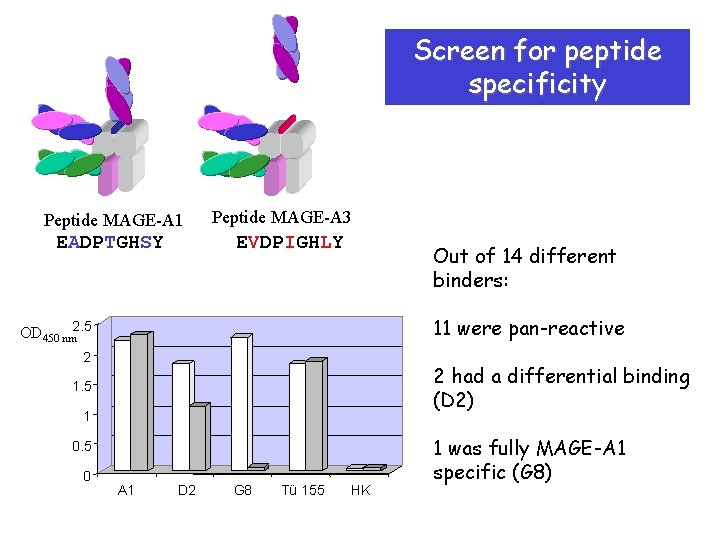
Screen for peptide specificity Peptide MAGE-A 1 EADPTGHSY Peptide MAGE-A 3 EVDPIGHLY Out of 14 different binders: 11 were pan-reactive OD 450 nm 2. 5 2 2 had a differential binding (D 2) 1. 5 1 0. 5 0 A 1 D 2 G 8 Tü 155 HK 1 was fully MAGE-A 1 specific (G 8)

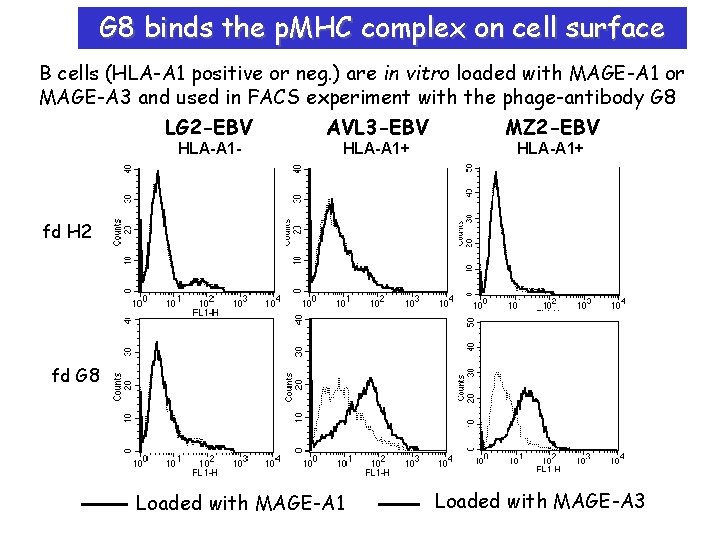
G 8 binds the p. MHC complex on cell surface B cells (HLA-A 1 positive or neg. ) are in vitro loaded with MAGE-A 1 or MAGE-A 3 and used in FACS experiment with the phage-antibody G 8 LG 2 -EBV AVL 3 -EBV MZ 2 -EBV HLA-A 1 - HLA-A 1+ fd H 2 fd G 8 Loaded with MAGE-A 1 Loaded with MAGE-A 3

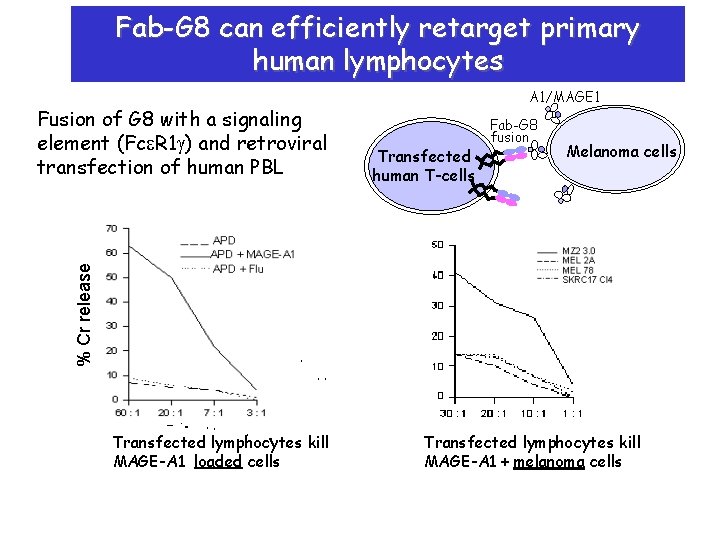
Fab-G 8 can efficiently retarget primary human lymphocytes Transfected human T-cells Fab-G 8 fusion Melanoma cells % Cr release Fusion of G 8 with a signaling element (Fce. R 1 g) and retroviral transfection of human PBL A 1/MAGE 1 Transfected lymphocytes kill MAGE-A 1 loaded cells Transfected lymphocytes kill MAGE-A 1 + melanoma cells

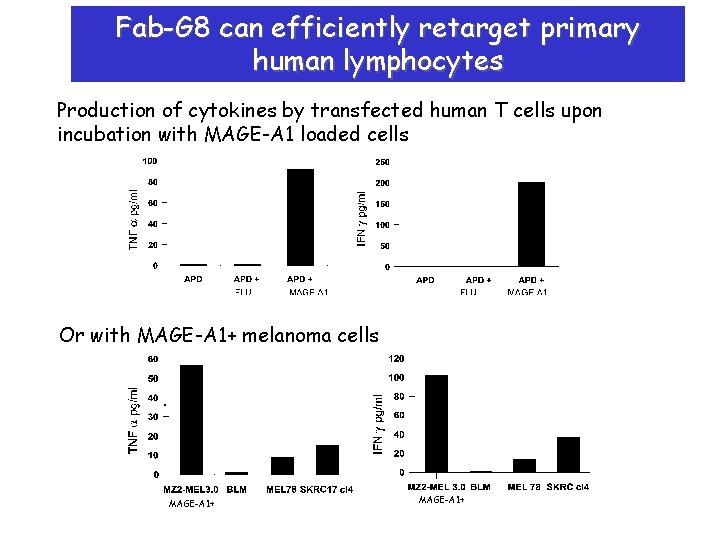
Fab-G 8 can efficiently retarget primary human lymphocytes Production of cytokines by transfected human T cells upon incubation with MAGE-A 1 loaded cells Or with MAGE-A 1+ melanoma cells MAGE-A 1+

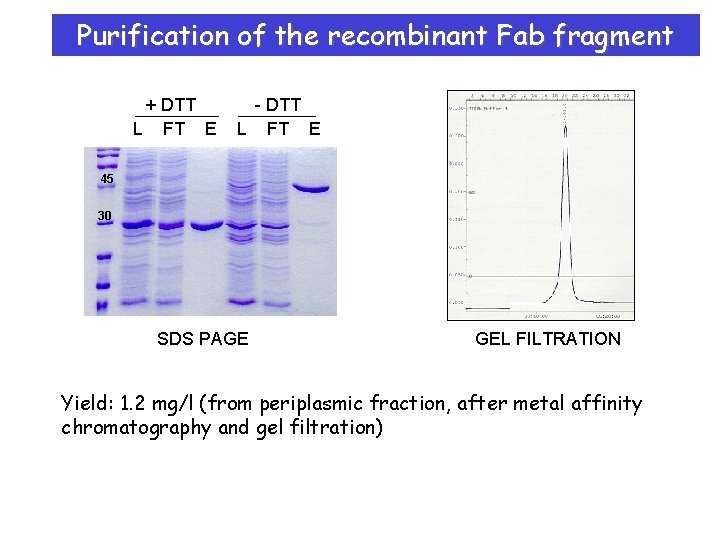
Purification of the recombinant Fab fragment + DTT L FT E - DTT L FT E 45 30 SDS PAGE GEL FILTRATION Yield: 1. 2 mg/l (from periplasmic fraction, after metal affinity chromatography and gel filtration)

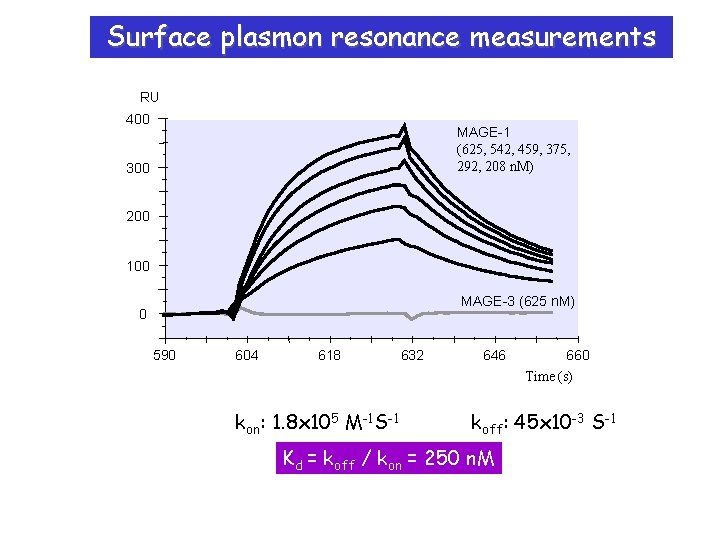
Surface plasmon resonance measurements RU 400 MAGE-1 (625, 542, 459, 375, 292, 208 n. M) 300 200 100 MAGE-3 (625 n. M) 0 590 604 618 632 646 660 Time (s) kon: 1. 8 x 105 M-1 S-1 koff: 45 x 10 -3 S-1 Kd = koff / kon = 250 n. M

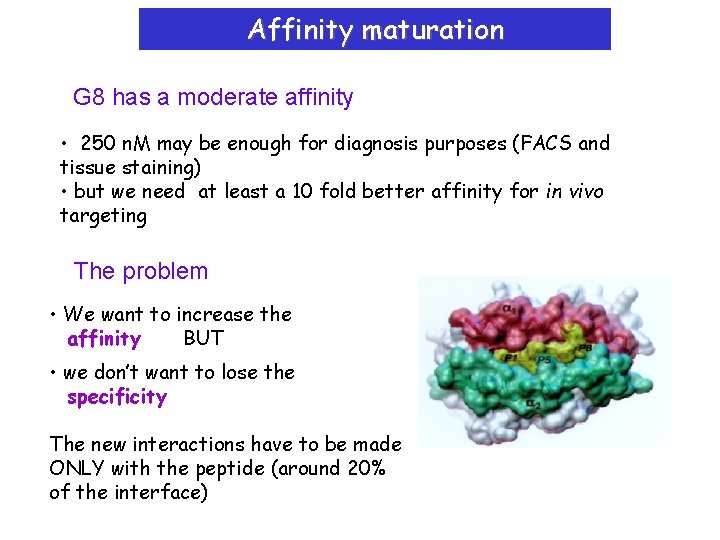
Affinity maturation G 8 has a moderate affinity • 250 n. M may be enough for diagnosis purposes (FACS and tissue staining) • but we need at least a 10 fold better affinity for in vivo targeting The problem • We want to increase the affinity BUT • we don’t want to lose the specificity The new interactions have to be made ONLY with the peptide (around 20% of the interface)

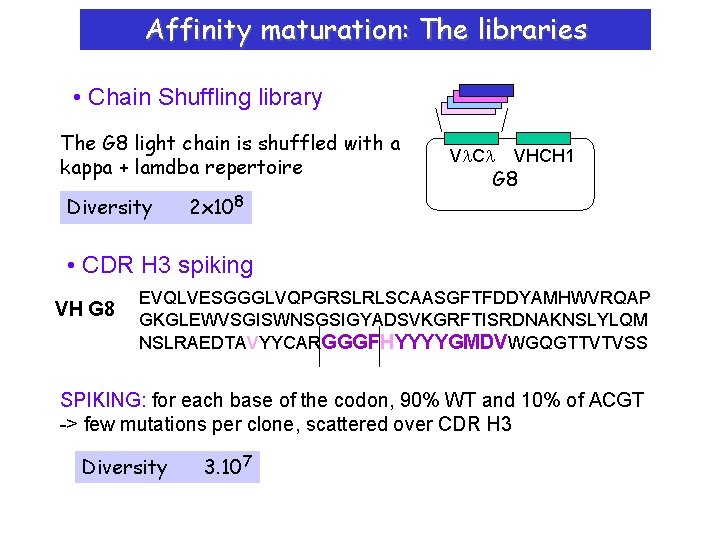
Affinity maturation: The libraries • Chain Shuffling library The G 8 light chain is shuffled with a kappa + lamdba repertoire Diversity 2 x 108 Vl. Cl VHCH 1 G 8 • CDR H 3 spiking VH G 8 EVQLVESGGGLVQPGRSLRLSCAASGFTFDDYAMHWVRQAP GKGLEWVSGISWNSGSIGYADSVKGRFTISRDNAKNSLYLQM NSLRAEDTAVYYCARGGGFHYYYYGMDVWGQGTTVTVSS SPIKING: for each base of the codon, 90% WT and 10% of ACGT -> few mutations per clone, scattered over CDR H 3 Diversity 3. 107

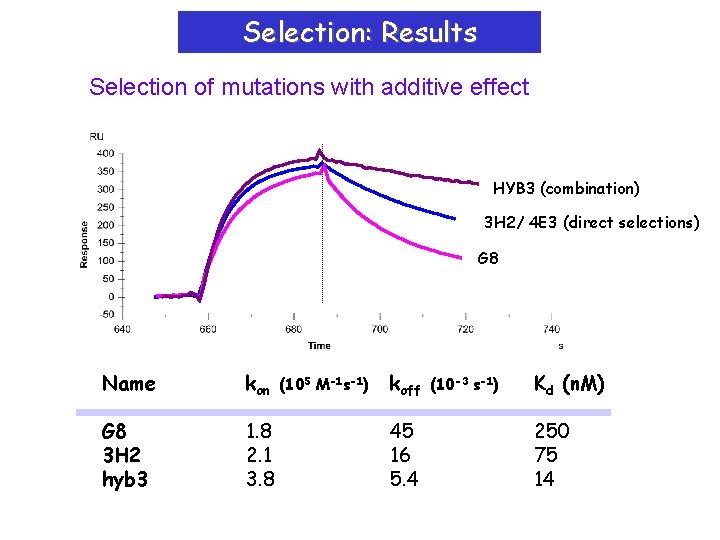
Selection: Results Selection of mutations with additive effect HYB 3 (combination) 3 H 2/ 4 E 3 (direct selections) G 8 Name kon G 8 3 H 2 hyb 3 1. 8 2. 1 3. 8 (105 M-1 s-1) koff 45 16 5. 4 (10 -3 s-1) Kd (n. M) 250 75 14

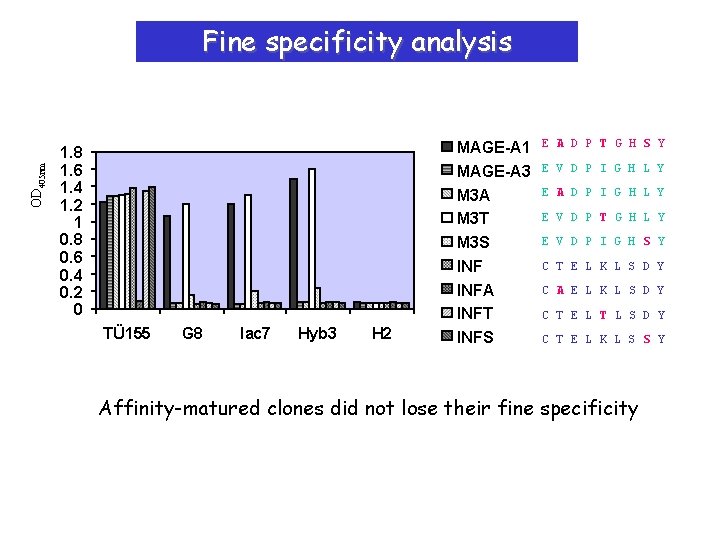
OD 405 nm Fine specificity analysis 1. 8 1. 6 1. 4 1. 2 1 0. 8 0. 6 0. 4 0. 2 0 TÜ 155 G 8 lac 7 Hyb 3 H 2 MAGE-A 1 MAGE-A 3 M 3 A M 3 T M 3 S INFA INFT INFS E A D P T G H S Y E V D P I G H L Y E A D P I G H L Y E V D P T G H L Y E V D P I G H S Y C T E L K L S D Y C A E L K L S D Y C T E L T L S D Y C T E L K L S S Y Affinity-matured clones did not lose their fine specificity

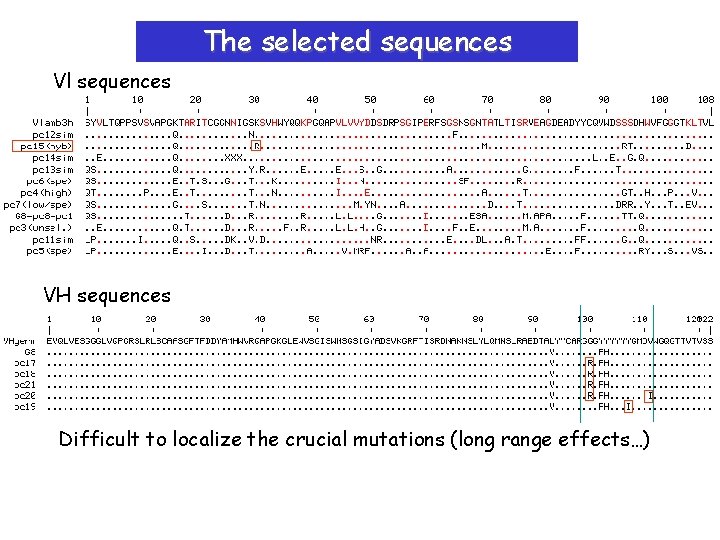
The selected sequences Vl sequences VH sequences Difficult to localize the crucial mutations (long range effects…)

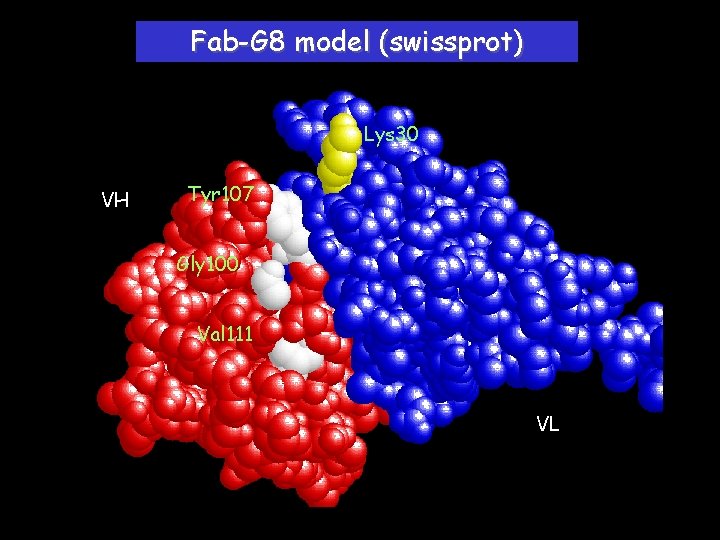
Fab-G 8 model (swissprot) Lys 30 VH Tyr 107 Gly 100 Val 111 VL

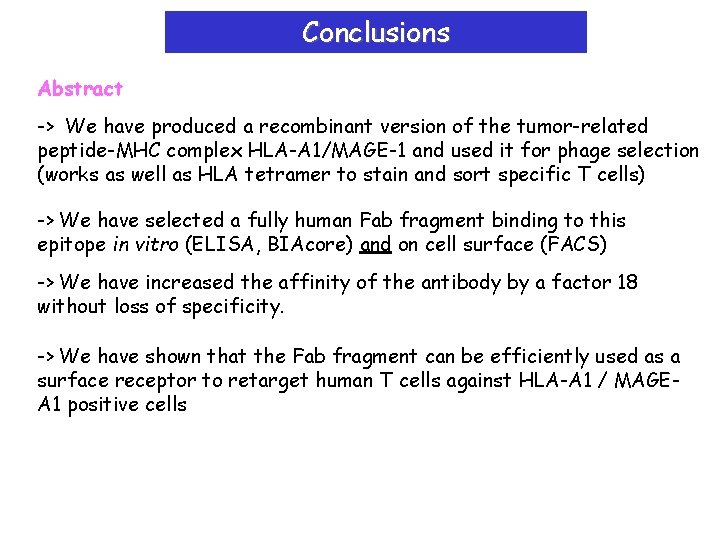
Conclusions Abstract -> We have produced a recombinant version of the tumor-related peptide-MHC complex HLA-A 1/MAGE-1 and used it for phage selection (works as well as HLA tetramer to stain and sort specific T cells) -> We have selected a fully human Fab fragment binding to this epitope in vitro (ELISA, BIAcore) and on cell surface (FACS) -> We have increased the affinity of the antibody by a factor 18 without loss of specificity. -> We have shown that the Fab fragment can be efficiently used as a surface receptor to retarget human T cells against HLA-A 1 / MAGEA 1 positive cells

Perspectives Crystallography -> G 8 and HLA-A 1/ MAGE-A 1 have been produced and purified in high amounts for crystallographic studies ->The p. MHC-Fab complex can be purified by gel filtration -> The high affinity version will also be produced
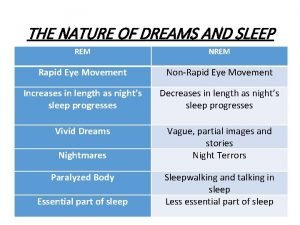
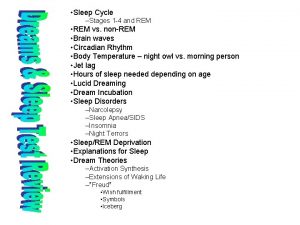
 Rem and non rem sleep
Rem and non rem sleep Standar ketebalan kanvas rem tromol
Standar ketebalan kanvas rem tromol Jennifer willemsen
Jennifer willemsen Vfbv
Vfbv Astrid karnebeek
Astrid karnebeek Mrk syndroom
Mrk syndroom Quintadeclinazione latino
Quintadeclinazione latino Prinsip kerja rem tromol
Prinsip kerja rem tromol Rem tene verba sequentur significato
Rem tene verba sequentur significato Stage 27 model sentences
Stage 27 model sentences Define rem
Define rem York norwegian study centre
York norwegian study centre Difference between sievert and roentgen
Difference between sievert and roentgen Primjer algoritma iz svakodnevnog života
Primjer algoritma iz svakodnevnog života Derechos reales y personales
Derechos reales y personales Rem blok tunggal
Rem blok tunggal Oktavijan
Oktavijan Varica rem narravit
Varica rem narravit Adults spend about ______% of their sleep in rem sleep.
Adults spend about ______% of their sleep in rem sleep. Palatin aventin
Palatin aventin Phenylketonuria (pku)
Phenylketonuria (pku) Actio publiciana in rem
Actio publiciana in rem Religion rem
Religion rem