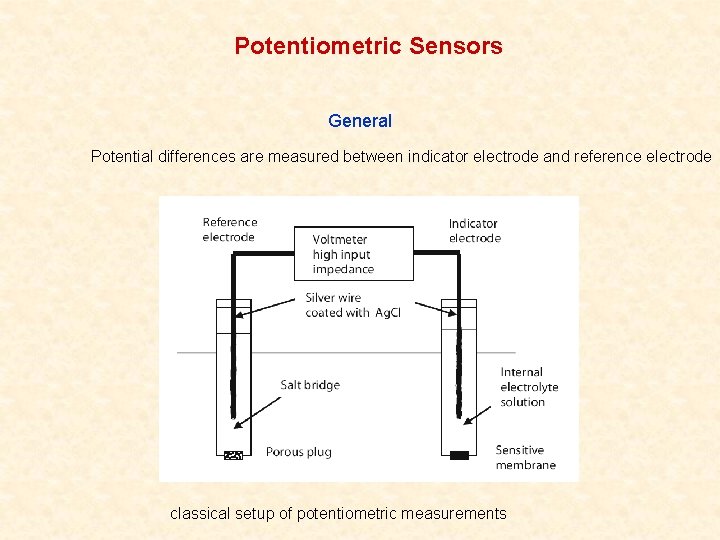
Potentiometric Sensors Potentiometric Sensors General Potential differences are



- Slides: 30

Potentiometric Sensors

Potentiometric Sensors General Potential differences are measured between indicator electrode and reference electrode classical setup of potentiometric measurements

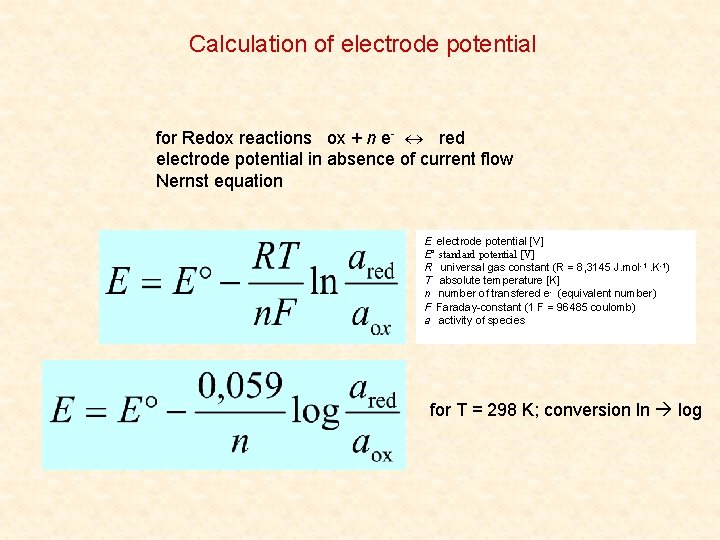
Calculation of electrode potential for Redox reactions ox + n e- red electrode potential in absence of current flow Nernst equation E electrode potential [V] E° standard potential [V] R universal gas constant (R = 8, 3145 J. mol-1. K-1) T absolute temperature [K] n number of transfered e- (equivalent number) F Faraday-constant (1 F = 96485 coulomb) a activity of species for T = 298 K; conversion ln log

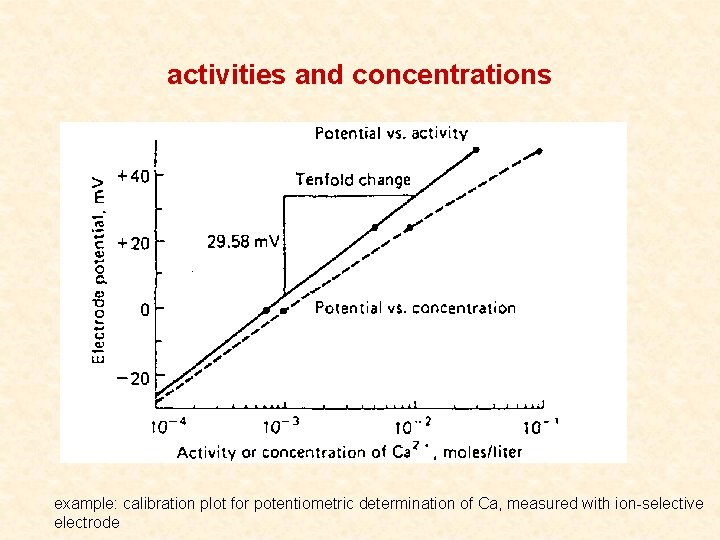
activities and concentrations example: calibration plot for potentiometric determination of Ca, measured with ion-selective electrode

Potentiometric Sensors mainly ion-selective (sensitive) electrodes, ISEs, or ISEs with an additional selective step e. g. , gas diffusion, enzymatic reaction Method measurement of cell potential in the absence of (relevant) currents indicator electrode (working electrode) half-cell with test solution potential dependent from one ionic species in solution reference electrode second half-cell with constant potential potentiometer voltmeter with high input impedance

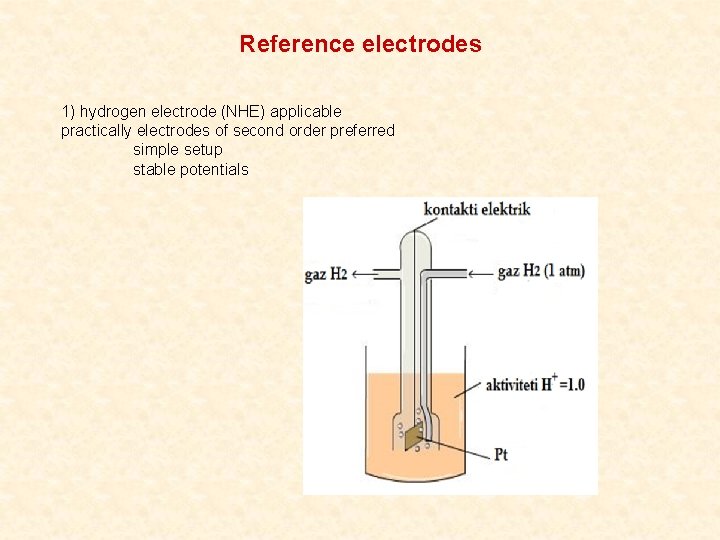
Reference electrodes 1) hydrogen electrode (NHE) applicable practically electrodes of second order preferred simple setup stable potentials

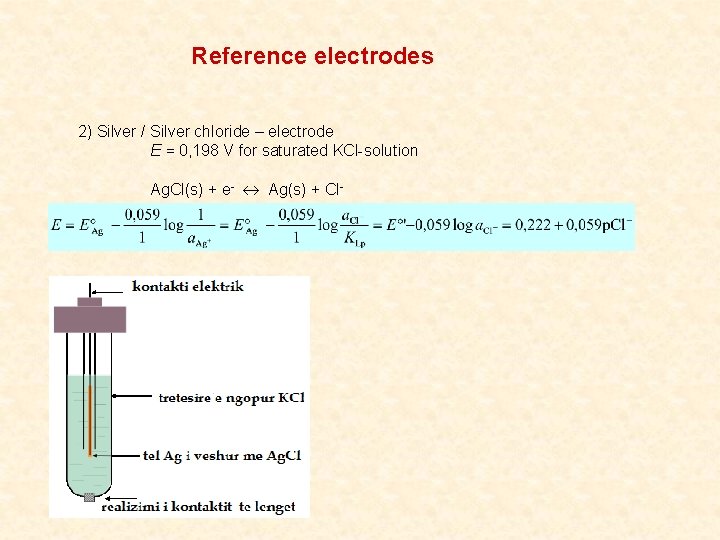
Reference electrodes 2) Silver / Silver chloride – electrode E = 0, 198 V for saturated KCl-solution Ag. Cl(s) + e- Ag(s) + Cl-

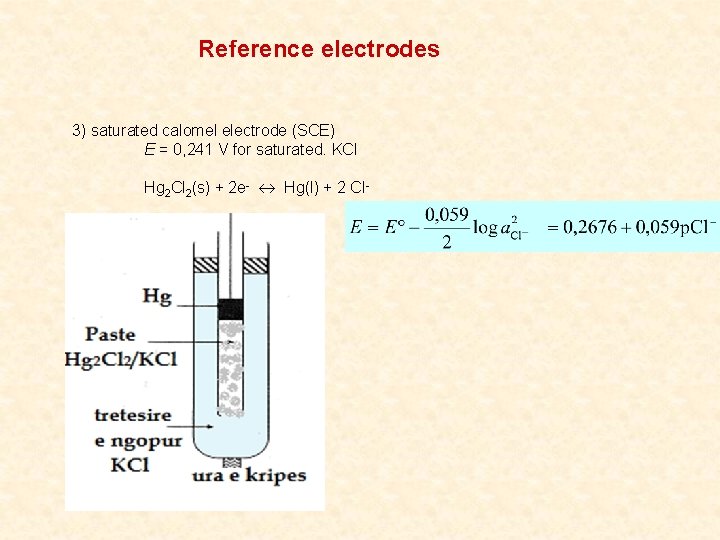
Reference electrodes 3) saturated calomel electrode (SCE) E = 0, 241 V for saturated. KCl Hg 2 Cl 2(s) + 2 e- Hg(l) + 2 Cl-

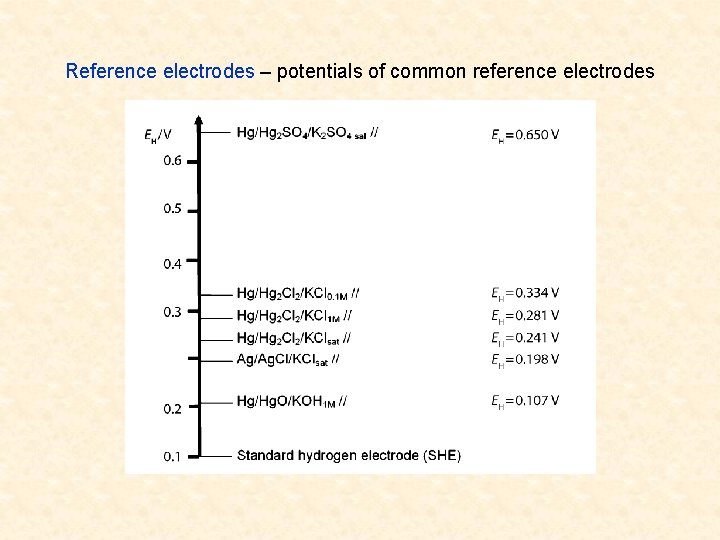
Reference electrodes – potentials of common reference electrodes

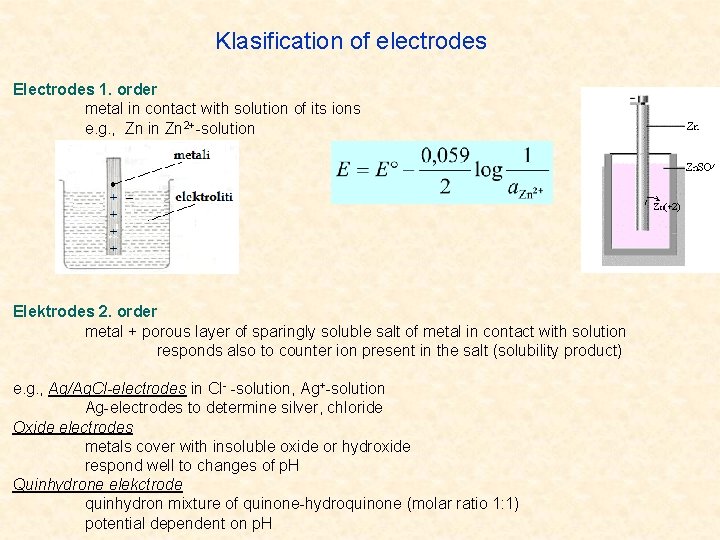
Klasification of electrodes Electrodes 1. order metal in contact with solution of its ions e. g. , Zn in Zn 2+-solution Elektrodes 2. order metal + porous layer of sparingly soluble salt of metal in contact with solution responds also to counter ion present in the salt (solubility product) e. g. , Ag/Ag. Cl-electrodes in Cl- -solution, Ag+-solution Ag-electrodes to determine silver, chloride Oxide electrodes metals cover with insoluble oxide or hydroxide respond well to changes of p. H Quinhydrone elekctrode quinhydron mixture of quinone-hydroquinone (molar ratio 1: 1) potential dependent on p. H

Redox electrodes inert electrodes (Pt, Au) measurement of püotentials of soluble, reversible redox pairs e. g. , Pt in a solution of Cr 3+ / Cr. O 42 - + 8 H+ +3 e- Cr 3+ + 4 H 2 O similarly: Fe 3+ /Fe 2+ detection limit of indicator electrodes: ca 10 -5 – 10 -6 mol/L

Indicator electrodes in potentiometry application selective electrodes potential corresponds mainly to activity of one ionic species in solution (should be) rather independent from the presence of other ions potential determining step is not a redox reaction exchange between membrane and electrolyte membrane potential characteristics of proper membranes - minimum solubility - electric conductivity (ionic migration within membrane) - selective reactivity with analyte Nernst equation applicable n = charge of the ionic species

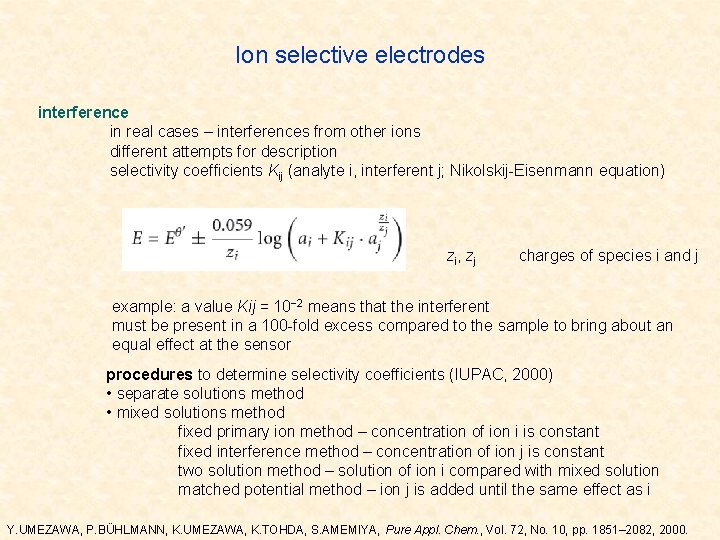
Ion selective electrodes interference in real cases – interferences from other ions different attempts for description selectivity coefficients Kij (analyte i, interferent j; Nikolskij-Eisenmann equation) zi, zj charges of species i and j example: a value Kij = 10− 2 means that the interferent must be present in a 100 -fold excess compared to the sample to bring about an equal effect at the sensor procedures to determine selectivity coefficients (IUPAC, 2000) • separate solutions method • mixed solutions method fixed primary ion method – concentration of ion i is constant fixed interference method – concentration of ion j is constant two solution method – solution of ion i compared with mixed solution matched potential method – ion j is added until the same effect as i Y. UMEZAWA, P. BÜHLMANN, K. UMEZAWA, K. TOHDA, S. AMEMIYA, Pure Appl. Chem. , Vol. 72, No. 10, pp. 1851– 2082, 2000.

Types of Ion Selective Electrodes (ISE) a) glass membrane for H+ and alkali metals b) solid state electrodes for halogenides, CN-, S 2 -, Cd 2+, Cu 2+, Pb 2+, Ag+ c) liquid membrane electrodes for K+, Ca 2+, NO 3 -, Cl. O 4 -. . . d) gas sensing electrodes for NH 3, SO 2 e) enzyme electrodes

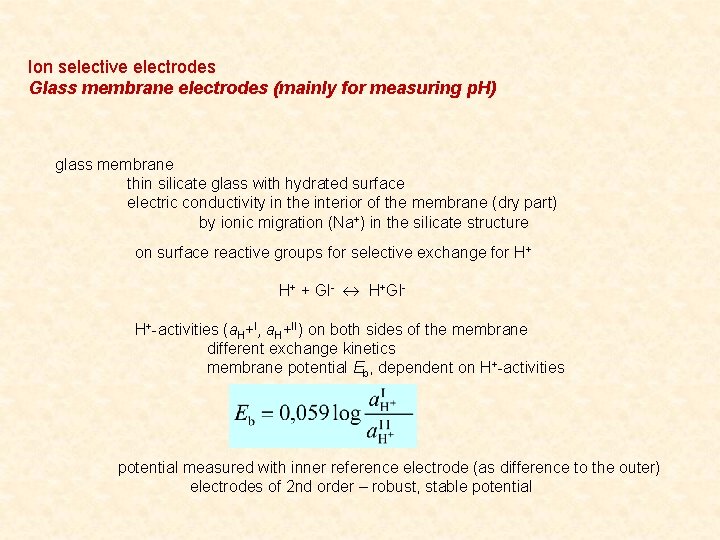
Ion selective electrodes Glass membrane electrodes (mainly for measuring p. H) glass membrane thin silicate glass with hydrated surface electric conductivity in the interior of the membrane (dry part) by ionic migration (Na+) in the silicate structure on surface reactive groups for selective exchange for H+ H+ + Gl- H+Gl. H+-activities (a. H+I, a. H+II) on both sides of the membrane different exchange kinetics membrane potential Eb, dependent on H+-activities potential measured with inner reference electrode (as difference to the outer) electrodes of 2 nd order – robust, stable potential

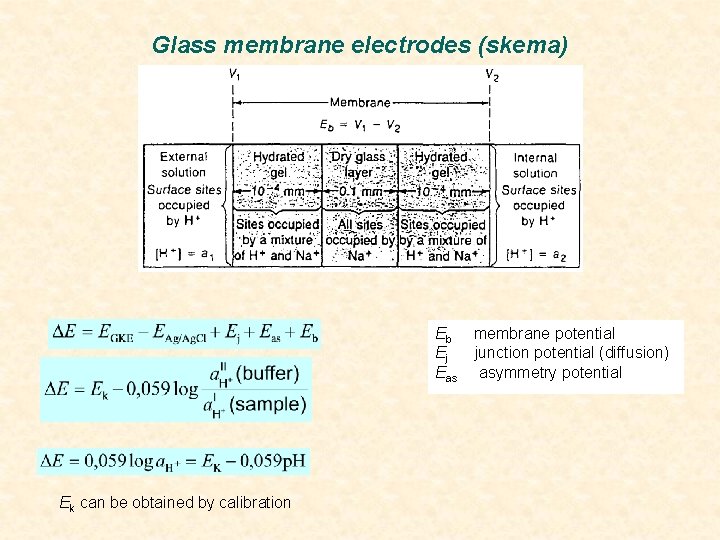
Glass membrane electrodes (skema) Eb Ej Eas Ek can be obtained by calibration membrane potential junction potential (diffusion) asymmetry potential

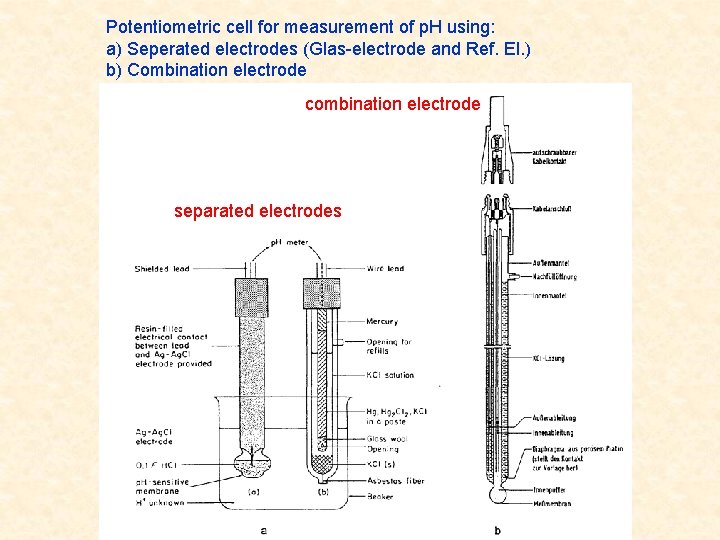
Potentiometric cell for measurement of p. H using: a) Seperated electrodes (Glas-electrode and Ref. El. ) b) Combination electrode combination electrode separated electrodes

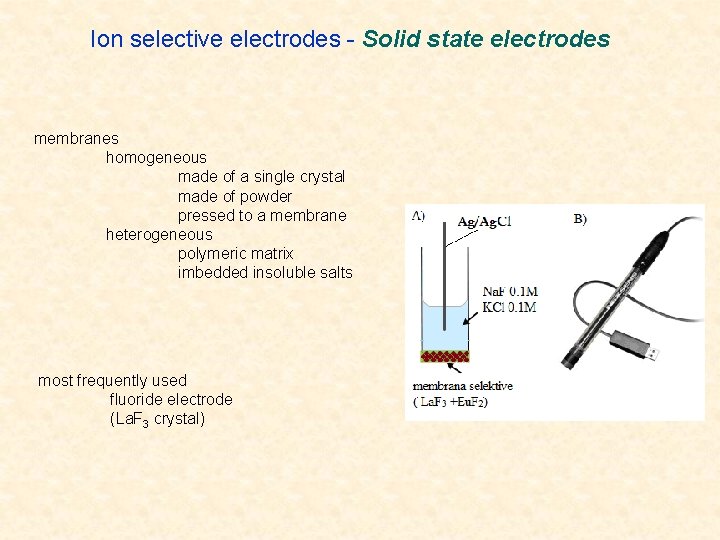
Ion selective electrodes - Solid state electrodes membranes homogeneous made of a single crystal made of powder pressed to a membrane heterogeneous polymeric matrix imbedded insoluble salts most frequently used fluoride electrode (La. F 3 crystal)

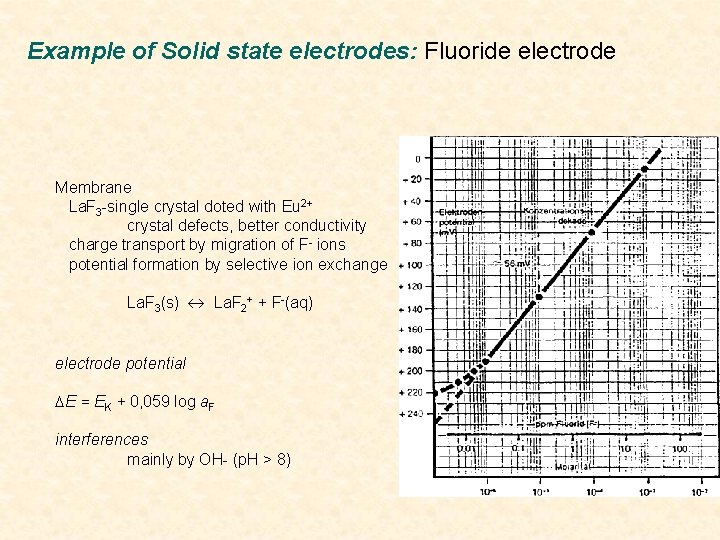
Example of Solid state electrodes: Fluoride electrode Membrane La. F 3 -single crystal doted with Eu 2+ crystal defects, better conductivity charge transport by migration of F- ions potential formation by selective ion exchange La. F 3(s) La. F 2+ + F-(aq) electrode potential DE = EK + 0, 059 log a. F interferences mainly by OH- (p. H > 8)

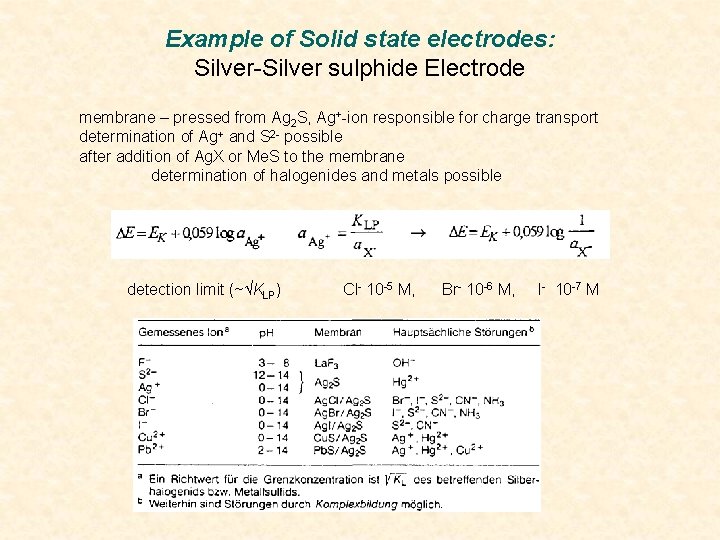
Example of Solid state electrodes: Silver-Silver sulphide Electrode membrane – pressed from Ag 2 S, Ag+-ion responsible for charge transport determination of Ag+ and S 2 - possible after addition of Ag. X or Me. S to the membrane determination of halogenides and metals possible detection limit (~ KLP) Cl- 10 -5 M, Br- 10 -6 M, I- 10 -7 M

Ion selective electrodes - Liquid membrane electrodes membrane from water-insoluble liquid ion exchanger impreganted on a porous support (clay or similar) ion exchanger reacts (more or less) selectively with target ion physiologically relevant ions can be determined Ca 2+, K+ (serum, blood) other analytes: BF 4 -, NO 3 -, Cl. O 4 - , hardness of water liquid membranes are also polymeric membranes (usually PVC) with a plasticizer („softener“) matrix behaves like a liquid ionophore embedded in membrane, reacts selectively with ion

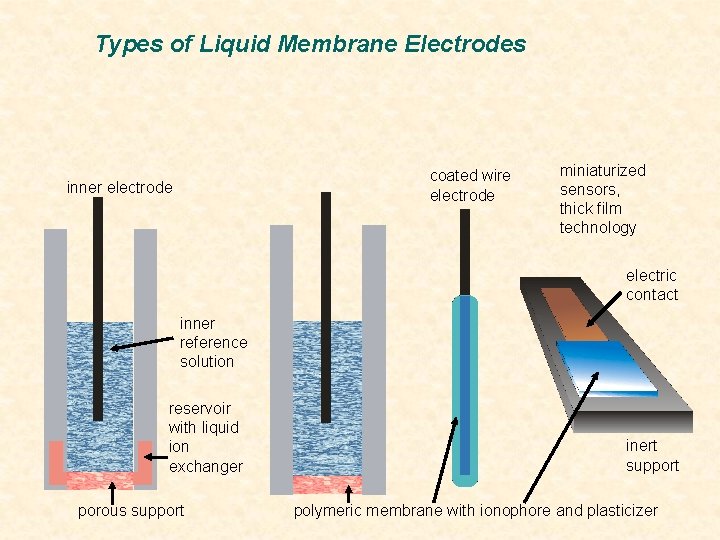
Types of Liquid Membrane Electrodes coated wire electrode inner electrode miniaturized sensors, thick film technology electric contact inner reference solution reservoir with liquid ion exchanger porous support inert support polymeric membrane with ionophore and plasticizer

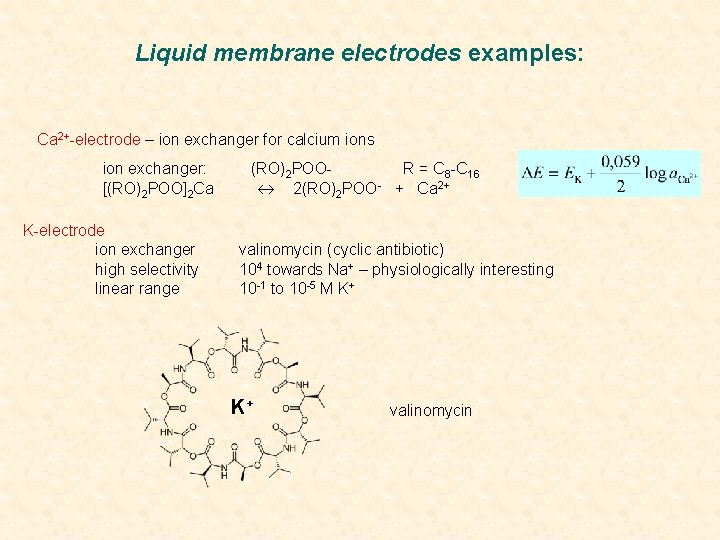
Liquid membrane electrodes examples: Ca 2+-electrode – ion exchanger for calcium ions ion exchanger: [(RO)2 POO]2 Ca K-electrode ion exchanger high selectivity linear range (RO)2 POOR = C 8 -C 16 2(RO)2 POO- + Ca 2+ valinomycin (cyclic antibiotic) 104 towards Na+ – physiologically interesting 10 -1 to 10 -5 M K+ K+ valinomycin

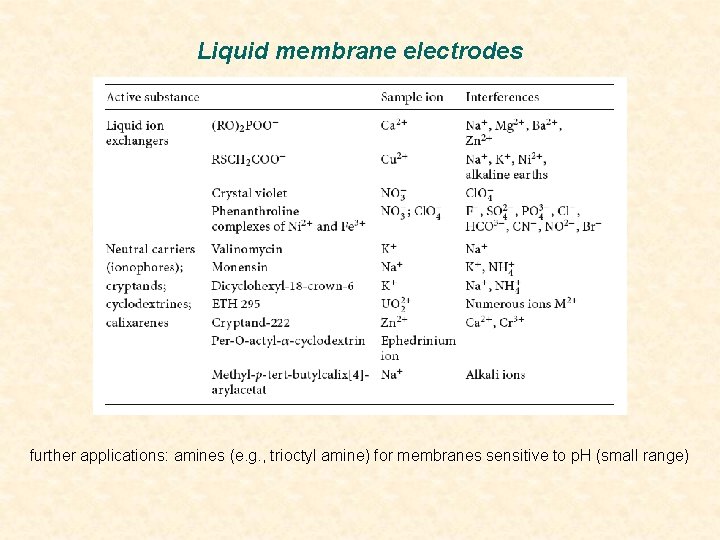
Liquid membrane electrodes further applications: amines (e. g. , trioctyl amine) for membranes sensitive to p. H (small range)

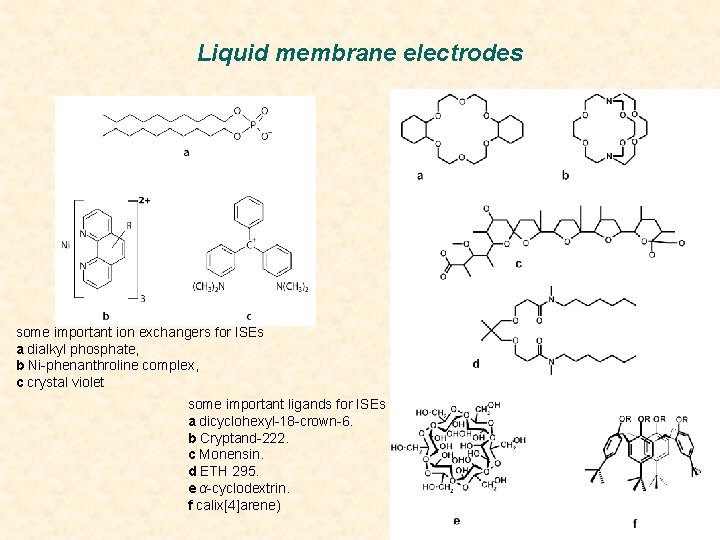
Liquid membrane electrodes some important ion exchangers for ISEs a dialkyl phosphate, b Ni-phenanthroline complex, c crystal violet some important ligands for ISEs a dicyclohexyl-18 -crown-6. b Cryptand-222. c Monensin. d ETH 295. e α-cyclodextrin. f calix[4]arene)

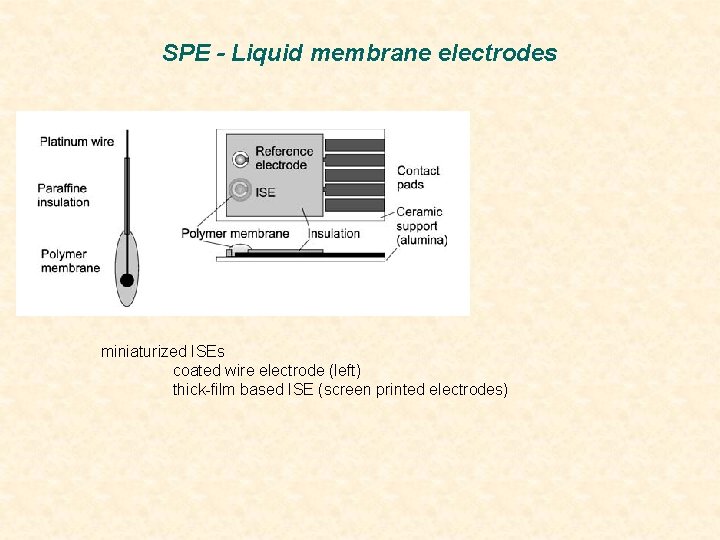
SPE - Liquid membrane electrodes miniaturized ISEs coated wire electrode (left) thick-film based ISE (screen printed electrodes)

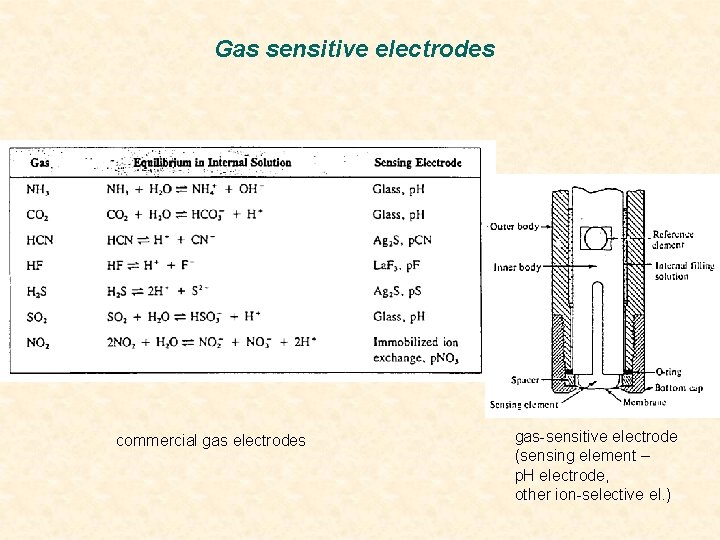
Ion selective electrodes -Gas sensitive electrodes Sensors for measuring gases (NH 3, CO 2, HCN) in gas phase or dissolved gas-permeable membrane gases diffuse from sample(solution) into space between membrane and ion-selective electrode equilibrium between outer and inner space after some seconds to minutes in inner space secondary effect measured (mainly p. H-value) proportional to gas concentration in the sample interference only by other (dissolved) gases gas-permeable membranes porous membranes - PTFE, PP (porosity: up to 70%, pore size: < 1 µm) water rejected by hydrophobic membranes gases permeate homogeneous membranes (e. g. silikone) gases are dissolved in membrane

Gas sensitive electrodes commercial gas electrodes gas-sensitive electrode (sensing element – p. H electrode, other ion-selective el. )

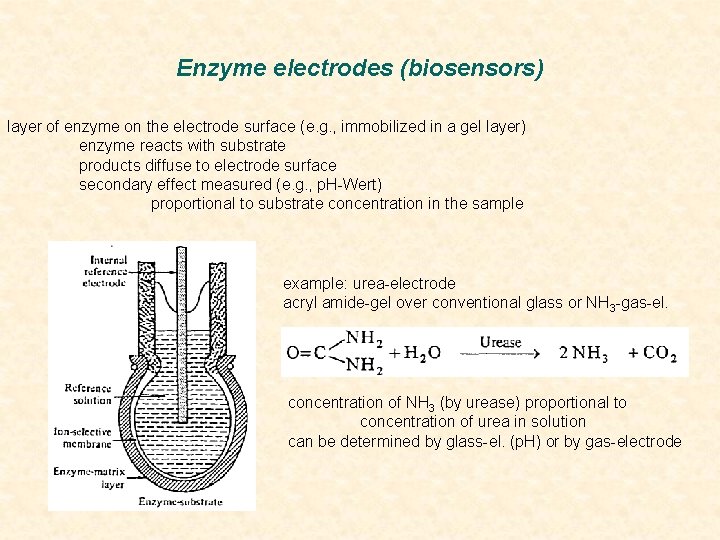
Enzyme electrodes (biosensors) layer of enzyme on the electrode surface (e. g. , immobilized in a gel layer) enzyme reacts with substrate products diffuse to electrode surface secondary effect measured (e. g. , p. H-Wert) proportional to substrate concentration in the sample example: urea-electrode acryl amide-gel over conventional glass or NH 3 -gas-el. concentration of NH 3 (by urease) proportional to concentration of urea in solution can be determined by glass-el. (p. H) or by gas-electrode

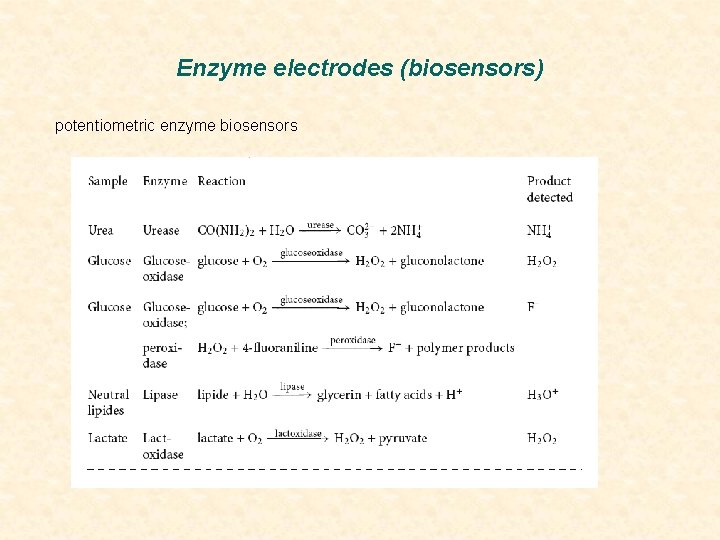
Enzyme electrodes (biosensors) potentiometric enzyme biosensors