Potential Ep H Diagram for H 2 O
- Slides: 6

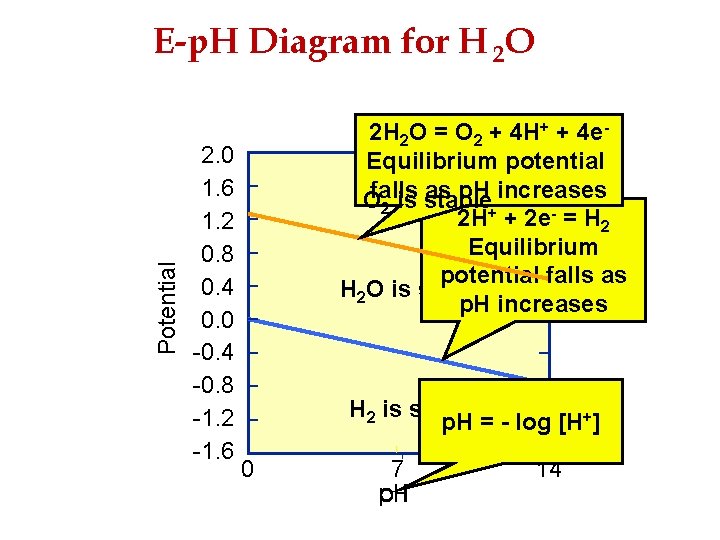
Potential E-p. H Diagram for H 2 O 2. 0 1. 6 1. 2 0. 8 0. 4 0. 0 -0. 4 -0. 8 -1. 2 -1. 6 2 H 2 O = O 2 + 4 H+ + 4 e. Equilibrium potential as p. H increases Ofalls is stable 2 2 H+ + 2 e- = H 2 Equilibrium potential falls as H 2 O is stable p. H increases H 2 is stable p. H = - log [H+] 0 7 14

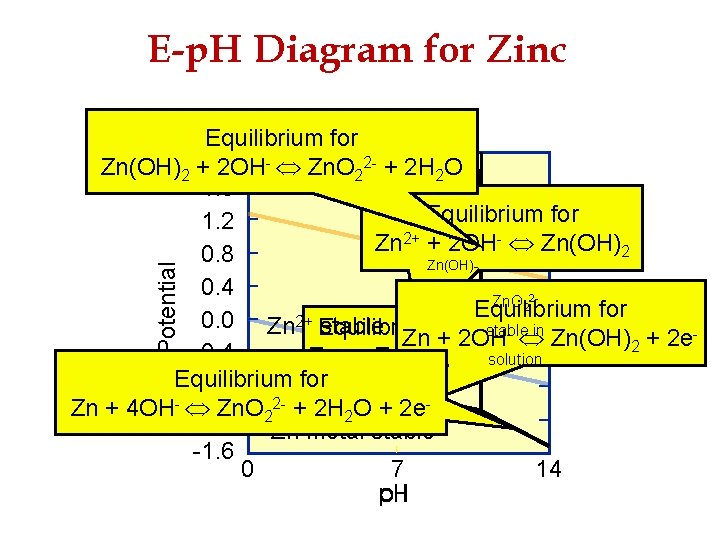
E-p. H Diagram for Zinc Potential Equilibrium for Zn(OH)2 +2. 0 2 OH- Zn. O 22 - + 2 H 2 O 1. 6 Equilibrium for 1. 2 2+ + 2 OH- Zn(OH) Zn 2 0. 8 Zn(OH) 0. 4 stable Zn. O 22 solid Equilibrium for 0. 0 Zn 2+ Equilibrium stable Zn +for stable in - 2 OH Zn(OH) + 2 e 2 -0. 4 Zn 2+ + 2 e- solution in solution Equilibrium for -0. 8 2 - + 2 H O + 2 e. Zn + 4 OH- Zn. O 2 2 -1. 2 Zn metal stable -1. 6 0 7 14 2

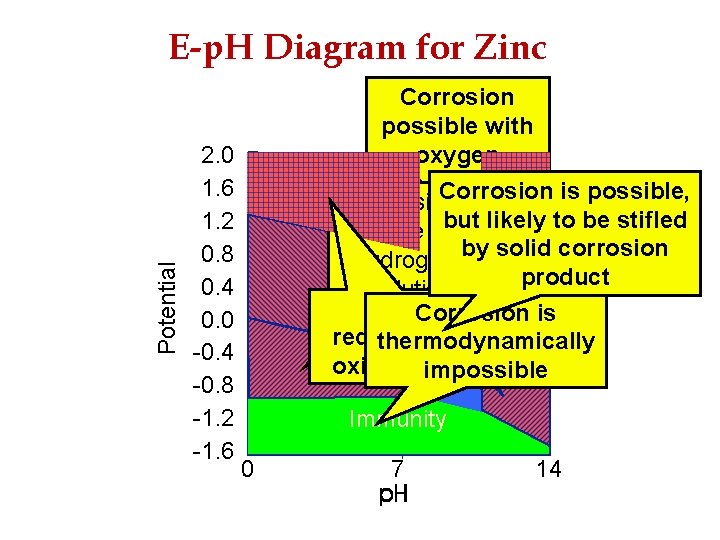
2 Corrosion 2. 0 1. 6 1. 2 0. 8 0. 4 0. 0 -0. 4 -0. 8 -1. 2 -1. 6 Corrosion possible with oxygen reduction Corrosion is possible, Corrosion but likely to be stifled possible with by solid corrosion hydrogen Zn(OH) Corrosion product stable evolution Zn. O 22 Corrosion solid Corrosion is 2+ Zn stable in requires strong thermodynamically solution oxidising agent in solution impossible Passivity Potential E-p. H Diagram for Zinc Immunity Zn metal stable 0 7 14

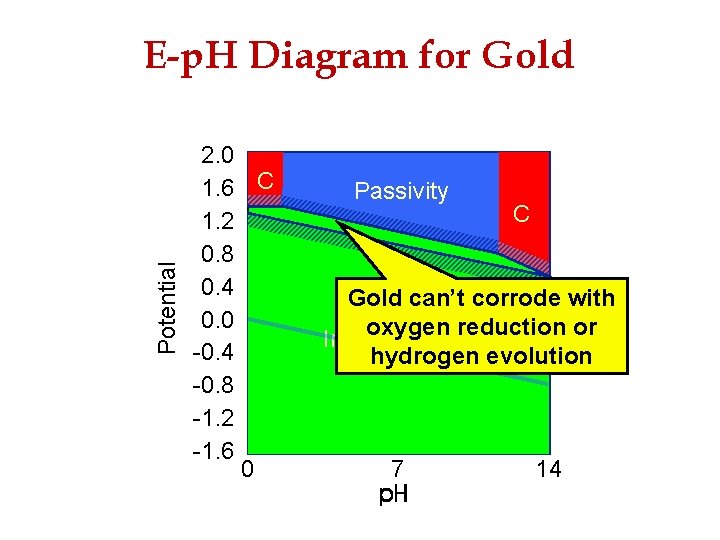
Potential E-p. H Diagram for Gold 2. 0 1. 6 C 1. 2 0. 8 0. 4 0. 0 -0. 4 -0. 8 -1. 2 -1. 6 0 Passivity C Gold can’t corrode with Gold metal stable oxygen reduction or Immunity hydrogen evolution 7 14

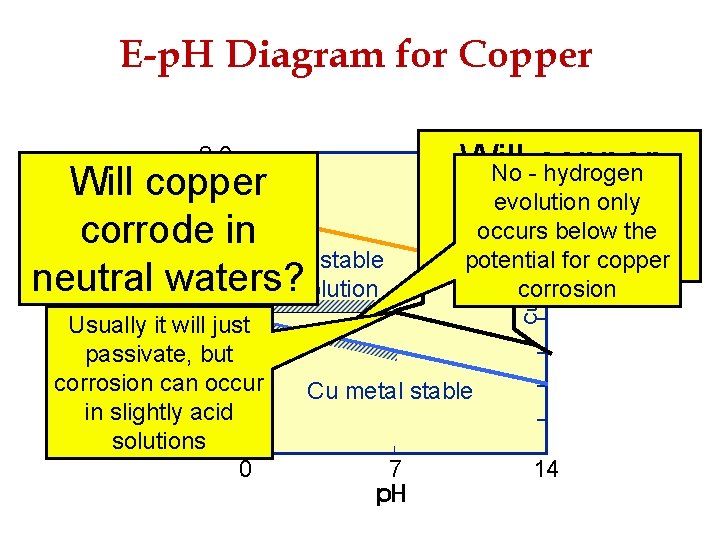
E-p. H Diagram for Copper Will copper corrode in acid? Potential Will copper corrode in neutral waters? Cu. O 22 - stable in soln. 2. 0 No - hydrogen 1. 6 evolution only Cu oxides 1. 2 occurs below the stable 0. 8 Cu 2+ stable potential for copper 0. 4 corrosion in solution Usually it will 0. 0 just passivate, -0. 4 but corrosion can-0. 8 occur Cu metal stable in slightly acid -1. 2 solutions -1. 6 0 7 14

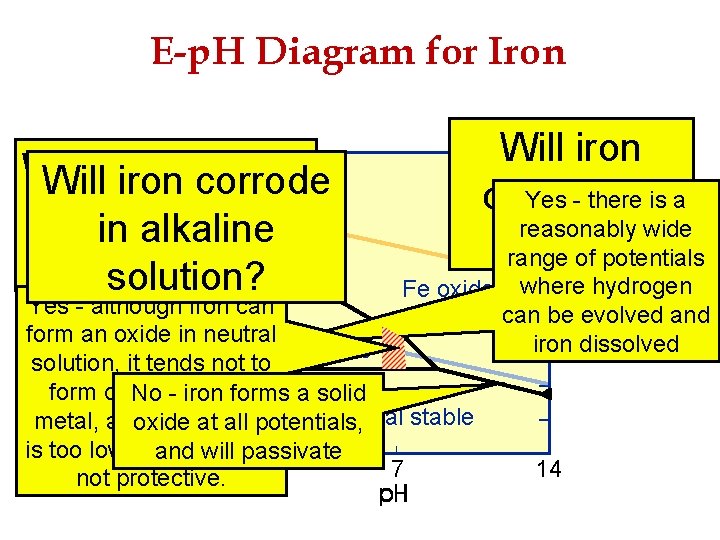
E-p. H Diagram for Iron Will iron corrode in acid? 2. 0 1. 6 Yes - there is a 1. 2 reasonably wide Fe 3+ 0. 8 range of potentials 0. 4 Fe oxides where hydrogen Yes - although iron can stable can be evolved and 0. 0 form an oxide in neutral 2+ iron dissolved Fe stable -0. 4 solution, it tends not to -0. 8 form directly on the No - iron forms a solid Fe metal stable -1. 2 metal, as oxide the potential at all potentials, is too low, therefore it is and-1. 6 will passivate 7 14 not protective. 0 Potential Willironcorrode ininneutral alkaline waters? solution?