Potential Energy Diagrams Burning Magnesium Exothermic Reaction Key
Potential Energy Diagrams

Burning Magnesium

Exothermic Reaction ______________
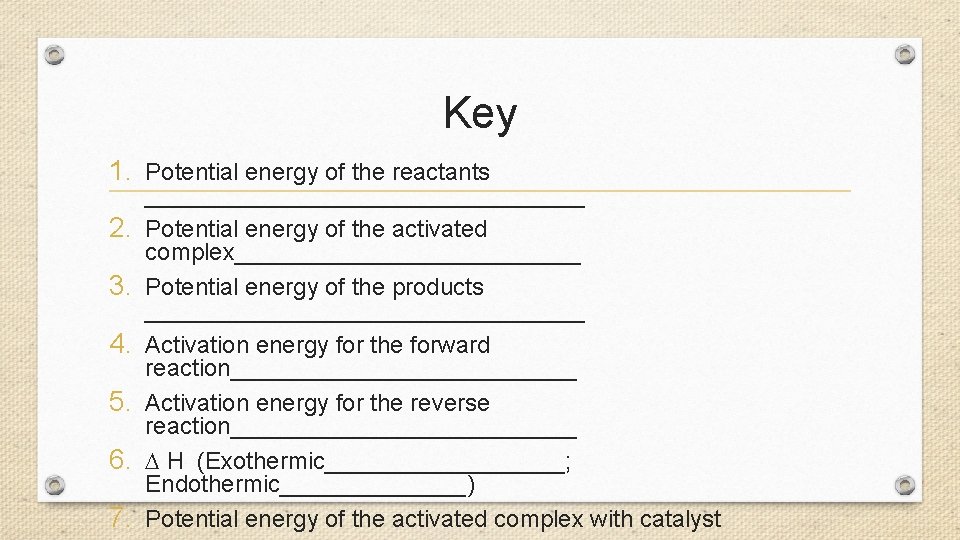
Key 1. Potential energy of the reactants 2. 3. 4. 5. 6. 7. _________________ Potential energy of the activated complex_____________ Potential energy of the products _________________ Activation energy for the forward reaction_____________ Activation energy for the reverse reaction_____________ H (Exothermic_________; Endothermic_______) Potential energy of the activated complex with catalyst
Endothermic Reaction • QUESTION: How would the potential energy diagram change for an endothermic reaction with and without a catalyst? • CLAIM: With your lab partner draw and label a potential energy diagram for the decomposition of magnesium oxide. Include your labeled diagram on the next slide.
Endothermic Reaction ___________
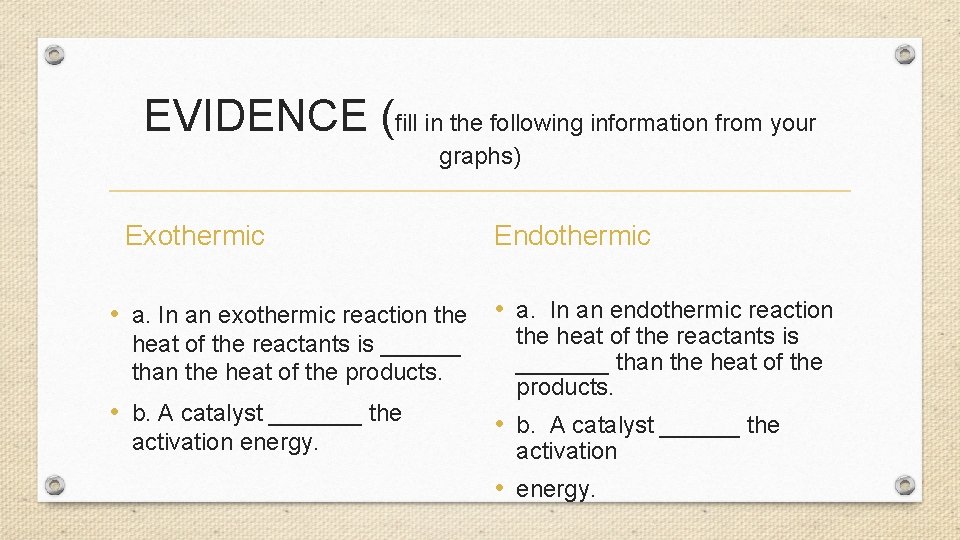
EVIDENCE (fill in the following information from your graphs) Exothermic Endothermic • a. In an exothermic reaction the • a. In an endothermic reaction heat of the reactants is ______ than the heat of the products. • b. A catalyst _______ the activation energy. the heat of the reactants is _______ than the heat of the products. • b. A catalyst ______ the activation • energy.
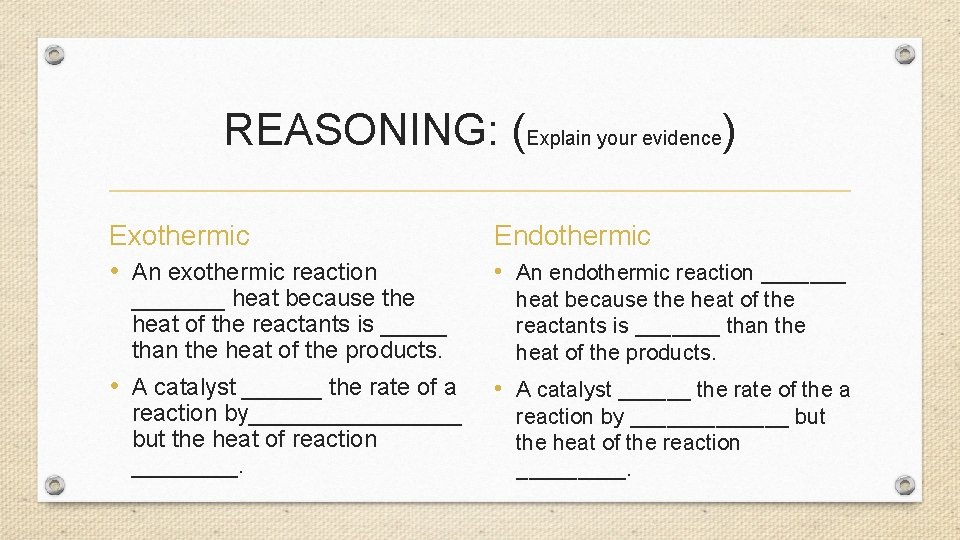
REASONING: (Explain your evidence) Exothermic • An exothermic reaction _______ heat because the heat of the reactants is _____ than the heat of the products. • A catalyst ______ the rate of a reaction by________ but the heat of reaction ____. Endothermic • An endothermic reaction _______ heat because the heat of the reactants is _______ than the heat of the products. • A catalyst ______ the rate of the a reaction by _______ but the heat of the reaction _____.

SUMMARY
- Slides: 9