Postpartum contraception Increasing patient access while maximizing resident










- Slides: 16

Postpartum contraception: Increasing patient access while maximizing resident education Julie Johnston MD Lawrence Family Medicine Residency Julia Mc. Donald DO, MPH Maine Dartmouth Family Medicine Residency

Disclosures Dr. Johnston • Merck – Nexplanon faculty trainer – Spouse with stock holdings • Johnson and Johnson – Spouse with stock holdings Dr. Mc. Donald-Nothing to disclose

Objectives Upon completion of this session, participants should be able to: 1. Identify safe and effective immediate postpartum contraceptive methods. 2. Name one barrier in your health setting that inhibits immediate postpartum contraceptive provision. 3. Develop a strategy for improving access to postpartum contraception through resident involvement.

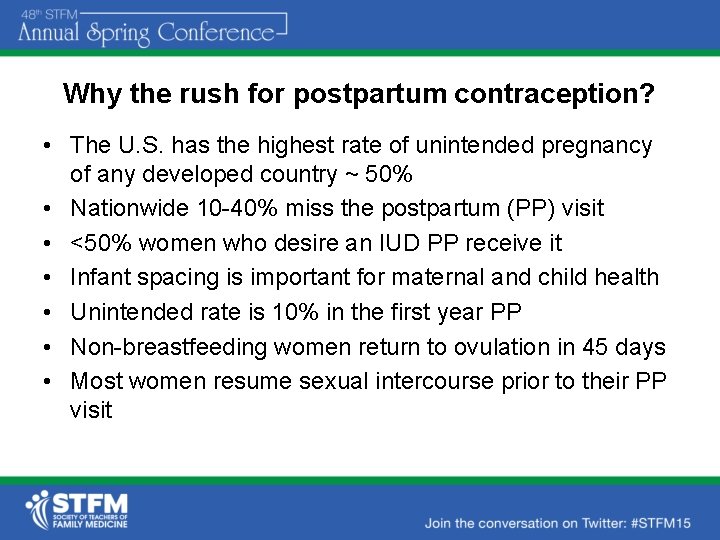
Why the rush for postpartum contraception? • The U. S. has the highest rate of unintended pregnancy of any developed country ~ 50% • Nationwide 10 -40% miss the postpartum (PP) visit • <50% women who desire an IUD PP receive it • Infant spacing is important for maternal and child health • Unintended rate is 10% in the first year PP • Non-breastfeeding women return to ovulation in 45 days • Most women resume sexual intercourse prior to their PP visit

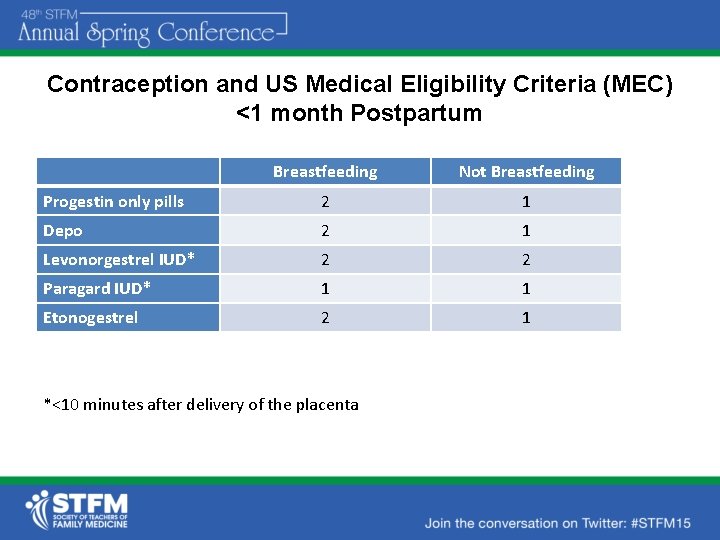
Contraception and US Medical Eligibility Criteria (MEC) <1 month Postpartum Breastfeeding Not Breastfeeding Progestin only pills 2 1 Depo 2 1 Levonorgestrel IUD* 2 2 Paragard IUD* 1 1 Etonogestrel 2 1 *<10 minutes after delivery of the placenta

U. S. Selected Practice Recommendations for Contraceptive Use, 2013 • Guidelines reflect the U. S. Medical Eligibility Criteria for immediate placement of IUDs and Implants postpartum

Women’s interest in IPP IUDs • Women may be more motivated in the immediate post-partum period to start contraception. • Women who agree to IPP placement are more than 10 x more likely to have the IUD inserted than those women who decided to wait until the post-partum visit Mohamed S. , Kamel, M. , Shaban O. , Salem, H. Acceptability for the use of postpartum intrauterine contraceptive devices: Assuit experience. Med Princ. Pract 2003; 12: 170 -5.

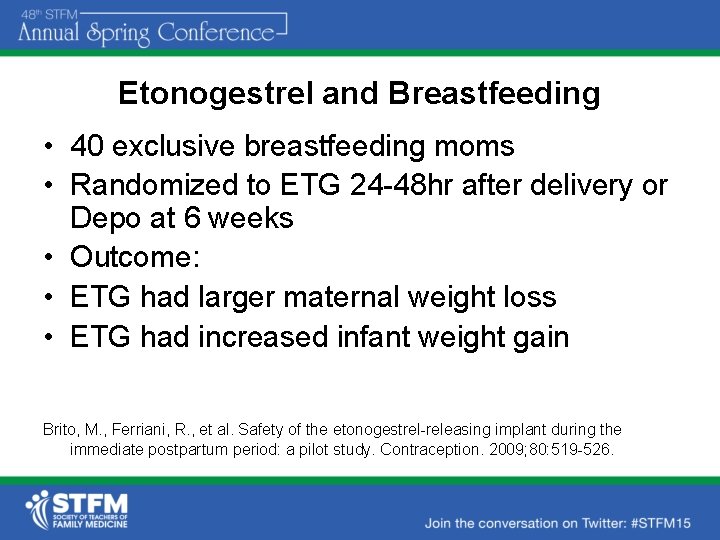
Etonogestrel and Breastfeeding • 40 exclusive breastfeeding moms • Randomized to ETG 24 -48 hr after delivery or Depo at 6 weeks • Outcome: • ETG had larger maternal weight loss • ETG had increased infant weight gain Brito, M. , Ferriani, R. , et al. Safety of the etonogestrel-releasing implant during the immediate postpartum period: a pilot study. Contraception. 2009; 80: 519 -526.

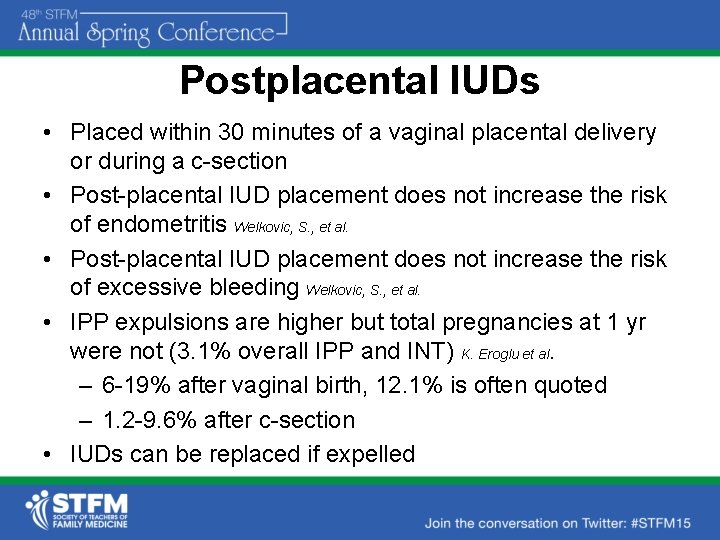
Postplacental IUDs • Placed within 30 minutes of a vaginal placental delivery or during a c-section • Post-placental IUD placement does not increase the risk of endometritis Welkovic, S. , et al. • Post-placental IUD placement does not increase the risk of excessive bleeding Welkovic, S. , et al. • IPP expulsions are higher but total pregnancies at 1 yr were not (3. 1% overall IPP and INT) K. Eroglu et al. – 6 -19% after vaginal birth, 12. 1% is often quoted – 1. 2 -9. 6% after c-section • IUDs can be replaced if expelled

What we did in Lawrence, MA • 2013: Initiated in-hospital post-partum ETG implant placement. • HOW: If ETG implant desired, ordered in EMR (device billed through GLFHC 403 b pharmacy), delivered to LGH pharmacy for verification, then brought to floor for (unbilled) placement. • Multiple benefits: — In women with ante partum stated intention to use, receipt of device (w/i 6 wks PP) INCREASED from 26. 5% to 93. 5% — Increased procedural numbers for residents — Communication between residents and staff (RN, lactation)

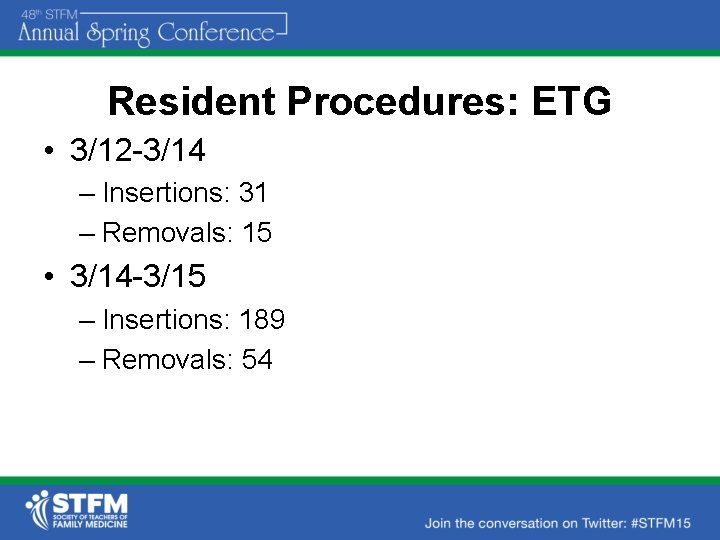
Resident Procedures: ETG • 3/12 -3/14 – Insertions: 31 – Removals: 15 • 3/14 -3/15 – Insertions: 189 – Removals: 54

LARC Funding Programs • RHEDI • Get LARC Now • Ryan (OB/GYN programs)

• • • Medicaid Coverage Alabama Colorado (+/-) Georgia Iowa Louisiana Maryland • • • Montana New Mexico New York Oklahoma South Carolina http: //www. acog. org/About-ACOG/ACOG-Departments/Long-Acting-Reversible. Contraception/Coding-and-Reimbursement-for-LARC/Reimbursement-Resources-for. Postpartum-LARC-Initiation/Medicaid-Reimbursement-for-Postpartum-LARC-By-State

Contacts • Alicia Luchowski at the ACOG LARC Program: ALuchowski@acog. org

• • • • References Celen, S. , Moroy, P. , Sucak, A. , Aktulay, A. , Danisman, N. Clinical outcomes of early postplacental insertion of intrauterine contraceptive devices. Contraception 2004; 69 279 -82. Chi, I. , Wilkens, L. , Rogers, S. Expulsions in Immediate Postpartum Insertions of Lippes Loop D and Copper T IUDs and their Counterpart Delta Devices-An epidemiological Analysis Contraception 1985 Aug; 32(2): 119 -134. Grimes, D. A. , Schulz, KF. , Van Vliet, HHAAM, Stanwood, NL. , Lopez, LM. Immediate post-partum insertion of intrauterine devices (Review). Cochrane Collaboration 2008 (3). J. L. Hayes et al. A pilot clinical trial of ultrasound-guided postplacental insertion of a levonorgestrel intrauterine device. Contraception 2007 Oct; 76(4): 292 -6. Welkovic, S. , et al. Post-partum bleeding and infection after post-placental IUD insertion. Contraception 2001; 63: 155 -9. K Eroglu et al. Comparison of efficacy and complications of IUD insertion in immediate postplacental/early post partum period with interval period: I year follow-up. Contraception 2006; 74(5): 376 -81. Mohamed S. , Kamel, M. , Shaban O. , Salem, H. Acceptability for the use of postpartum intrauterine contraceptive devices: Assuit experience. Med Princ. Pract 2003; 12: 170 -5. Muller, A. , et al. Transvaginal ultrasonographic assessment of the expulsion rate of intrauterine devices inserted in the immediate postpartum period: a pilot study. Contraception 2005; 72: 192 -195. Gurtcheff, S, MS; Turok, D. , et. al. Lactogenesis After Early Postpartum Use of the Contraceptive Implant: A Randomized Controlled Trial. Obstetrics and Gynecology 2011; 117: 1114 -1121. Kapp N, Curtis K, Nanda K. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception 2010; 82: 17– 37. Chen, B. , Reeves, M. Postplacental or delayed levonorgestrel intrauterine device insertion and breastfeeding duration. Contraception. 2011; 84(5): 499 -504. Stevermer, J. , Jones, K. , Egan, M. Offer this contraceptive to breastfeeding new moms. J Fam Pract. 2011; 60(12): 744 -746. Brito, M. , Ferriani, R. , et al. Safety of the etonogestrel-releasing implant during the immediate postpartum period: a pilot study. Contraception. 2009; 80: 519 -526.

Please evaluate this session at: stfm. org/sessionevaluation