PostOperative Anemia What is the Appropriate Transfusion Threshold










- Slides: 26

Post-Operative Anemia: What is the Appropriate Transfusion Threshold?

Objectives • Describe the physiology of oxygen delivery • List risks and benefits of red blood cell transfusions • Discuss the evidence regarding thresholds for red blood cell transfusion • Explain methods to prevent the need for intra- and post-operative transfusions

Case • A 14 year old female with adolescent idiopathic scoliosis is POD 2 after posterior spinal fusion. She is otherwise healthy. You are co-managing her care with the orthopaedic surgery team. • In the OR her EBL was 1 L, and she received 5 L of crystalloid, 500 m. L of albumin, and 400 m. L of cell saver blood. Her hemoglobin levels have been as follows: - Pre-op: 12. 5 - Immediately post-op: 8. 6 - POD 1: 7. 8 - POD 2: 7. 2 • Her post-operative course has been unremarkable so far except for intermittent tachycardia and difficult to control pain. On POD 2, the orthopaedic surgery team asks you whether a red blood cell transfusion is indicated for this patient. • The content of this module should help you provide an evidence-based recommendation for this patient.

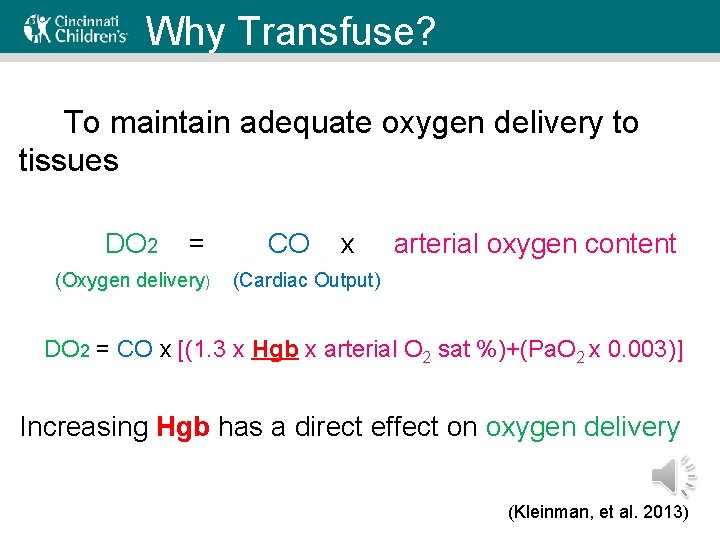
Why Transfuse? To maintain adequate oxygen delivery to tissues DO 2 = (Oxygen delivery) CO x arterial oxygen content (Cardiac Output) DO 2 = CO x [(1. 3 x Hgb x arterial O 2 sat %)+(Pa. O 2 x 0. 003)] Increasing Hgb has a direct effect on oxygen delivery (Kleinman, et al. 2013)

Compensation • In healthy patients at rest oxygen delivery is 4 x greater than oxygen consumption • Theoretically, for an adult, if intravascular volume and cardiovascular function are preserved, oxygen delivery is adequate until hematocrit is <10% (hgb <3. 5 g/d. L). This is due to: – increase cardiac output – rightward shift of the oxygen-hgb dissociation curve – increased oxygen extraction (Kleinman, et al. 2013)

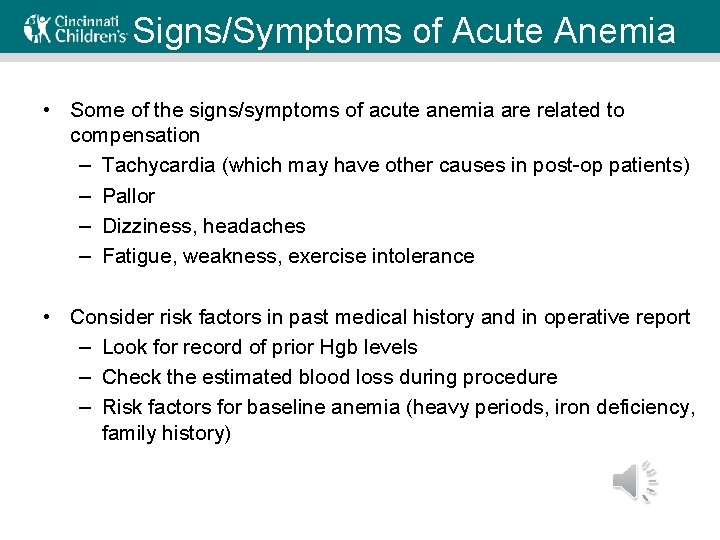
Signs/Symptoms of Acute Anemia • Some of the signs/symptoms of acute anemia are related to compensation – Tachycardia (which may have other causes in post-op patients) – Pallor – Dizziness, headaches – Fatigue, weakness, exercise intolerance • Consider risk factors in past medical history and in operative report – Look for record of prior Hgb levels – Check the estimated blood loss during procedure – Risk factors for baseline anemia (heavy periods, iron deficiency, family history)

Risk of not Transfusing Mortality in adult patients with very low postoperative Hgb levels who declined blood transfusion (adapted from Carson, et al. 2002)

Risks of Transfusing Potential Transfusion Complications • Risk of infection following transfusion of whole blood or red blood cell products Infection Risk Hepatitis B 1: 58, 000 to 1: 269, 000 Hepatitis C 1: 1, 000 to 1: 2, 000 Human Immunodeficiency Virus (HIV) 1: 1, 500, 000 to 1: 2, 000 Human T-Cell Lymphotropic Virus (HTLV) 1: 1, 900, 000 (Teruya, 2013)

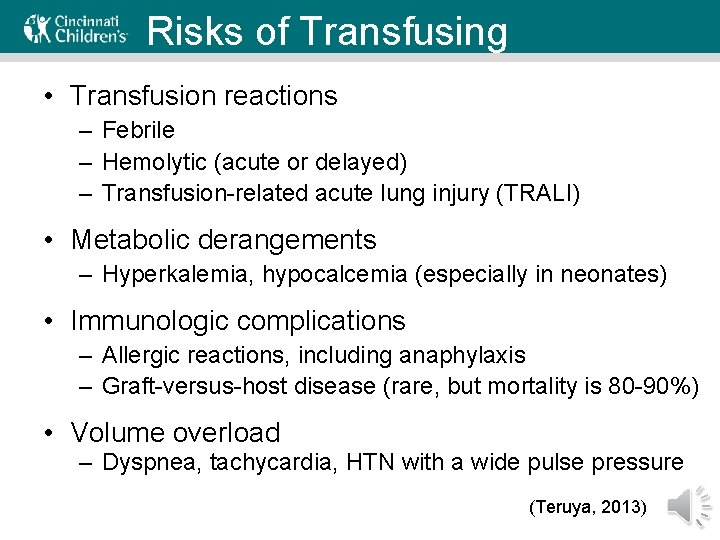
Risks of Transfusing • Transfusion reactions – Febrile – Hemolytic (acute or delayed) – Transfusion-related acute lung injury (TRALI) • Metabolic derangements – Hyperkalemia, hypocalcemia (especially in neonates) • Immunologic complications – Allergic reactions, including anaphylaxis – Graft-versus-host disease (rare, but mortality is 80 -90%) • Volume overload – Dyspnea, tachycardia, HTN with a wide pulse pressure (Teruya, 2013)

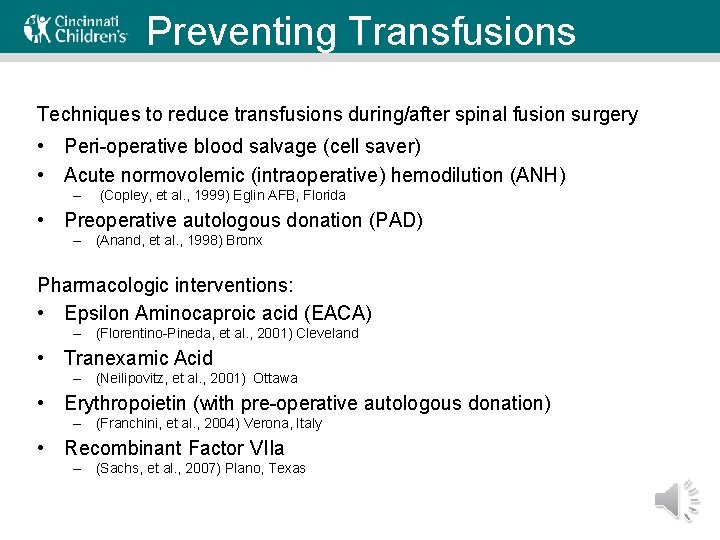
Preventing Transfusions Techniques to reduce transfusions during/after spinal fusion surgery • Peri-operative blood salvage (cell saver) • Acute normovolemic (intraoperative) hemodilution (ANH) – (Copley, et al. , 1999) Eglin AFB, Florida • Preoperative autologous donation (PAD) – (Anand, et al. , 1998) Bronx Pharmacologic interventions: • Epsilon Aminocaproic acid (EACA) – (Florentino-Pineda, et al. , 2001) Cleveland • Tranexamic Acid – (Neilipovitz, et al. , 2001) Ottawa • Erythropoietin (with pre-operative autologous donation) – (Franchini, et al. , 2004) Verona, Italy • Recombinant Factor VIIa – (Sachs, et al. , 2007) Plano, Texas

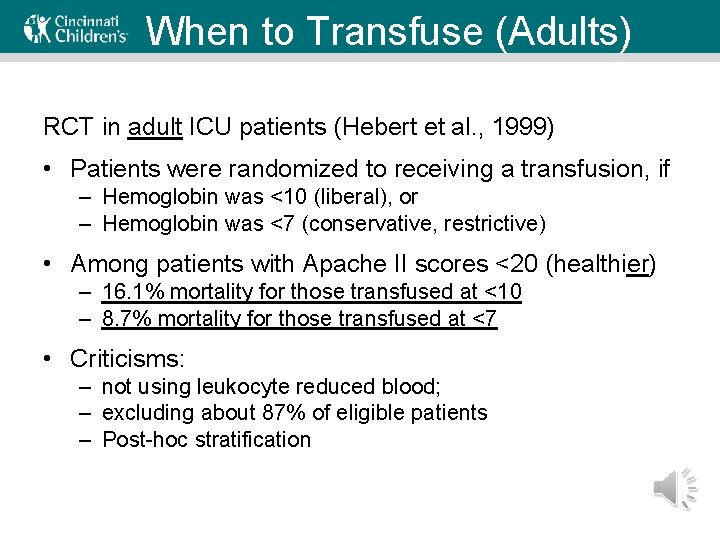
When to Transfuse (Adults) RCT in adult ICU patients (Hebert et al. , 1999) • Patients were randomized to receiving a transfusion, if – Hemoglobin was <10 (liberal), or – Hemoglobin was <7 (conservative, restrictive) • Among patients with Apache II scores <20 (healthier) – 16. 1% mortality for those transfused at <10 – 8. 7% mortality for those transfused at <7 • Criticisms: – not using leukocyte reduced blood; – excluding about 87% of eligible patients – Post-hoc stratification

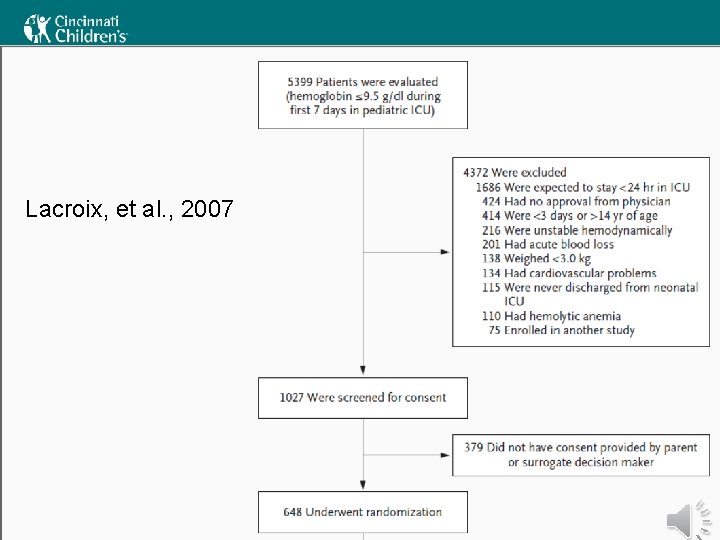
When to Transfuse (Kids) • • Recommendations vary somewhat Good evidence is scarce Recommendations are based mostly on expert opinion Some address children < 4 months and > 4 months of age separately • The best evidence about transfusion thresholds comes from the TRIPICU study From La. Croix, et al. (2007) and Rouette, et al. (2010)

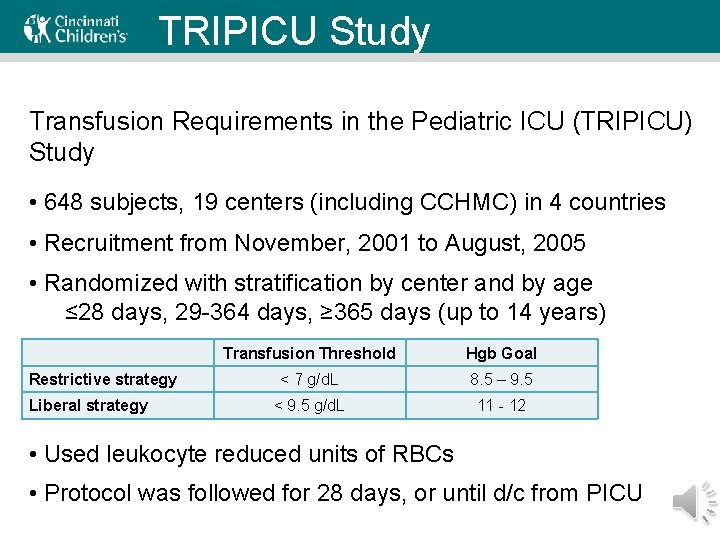
TRIPICU Study Transfusion Requirements in the Pediatric ICU (TRIPICU) Study • 648 subjects, 19 centers (including CCHMC) in 4 countries • Recruitment from November, 2001 to August, 2005 • Randomized with stratification by center and by age ≤ 28 days, 29 -364 days, ≥ 365 days (up to 14 years) Restrictive strategy Liberal strategy Transfusion Threshold Hgb Goal < 7 g/d. L 8. 5 – 9. 5 < 9. 5 g/d. L 11 - 12 • Used leukocyte reduced units of RBCs • Protocol was followed for 28 days, or until d/c from PICU

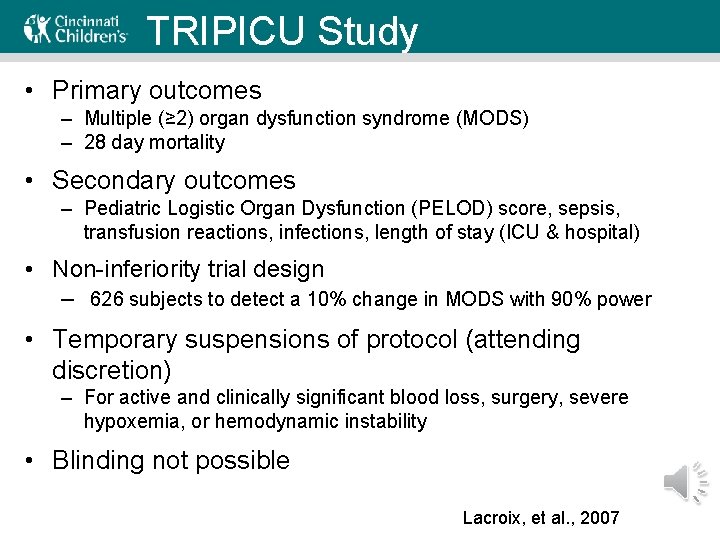
TRIPICU Study • Primary outcomes – Multiple (≥ 2) organ dysfunction syndrome (MODS) – 28 day mortality • Secondary outcomes – Pediatric Logistic Organ Dysfunction (PELOD) score, sepsis, transfusion reactions, infections, length of stay (ICU & hospital) • Non-inferiority trial design – 626 subjects to detect a 10% change in MODS with 90% power • Temporary suspensions of protocol (attending discretion) – For active and clinically significant blood loss, surgery, severe hypoxemia, or hemodynamic instability • Blinding not possible Lacroix, et al. , 2007

Lacroix, et al. , 2007

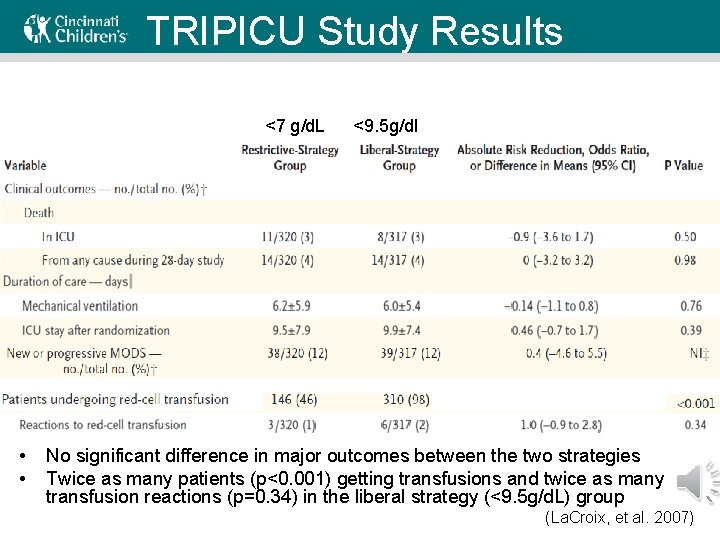
TRIPICU Study Results <7 g/d. L • • <9. 5 g/dl No significant difference in major outcomes between the two strategies Twice as many patients (p<0. 001) getting transfusions and twice as many transfusion reactions (p=0. 34) in the liberal strategy (<9. 5 g/d. L) group (La. Croix, et al. 2007)

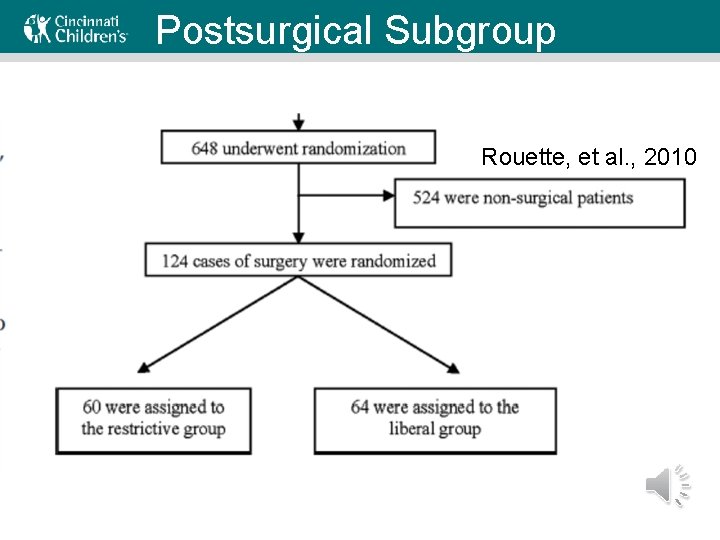
Postsurgical Subgroup Rouette, et al. , 2010

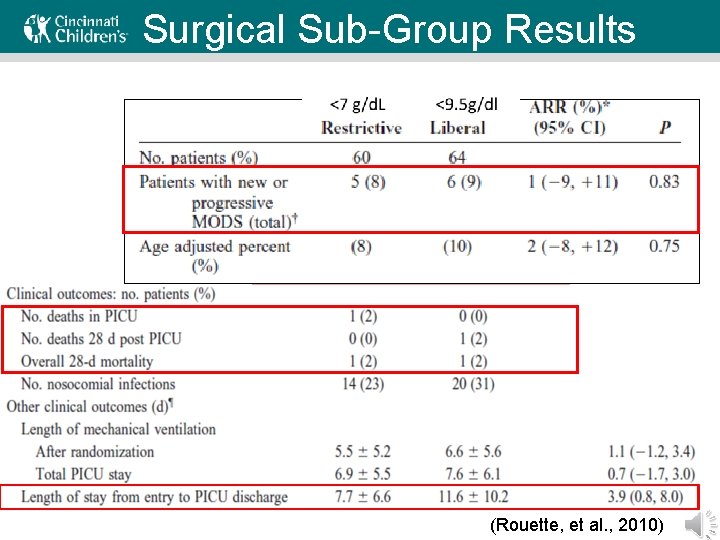
Surgical Sub-Group Results (Rouette, et al. , 2010)

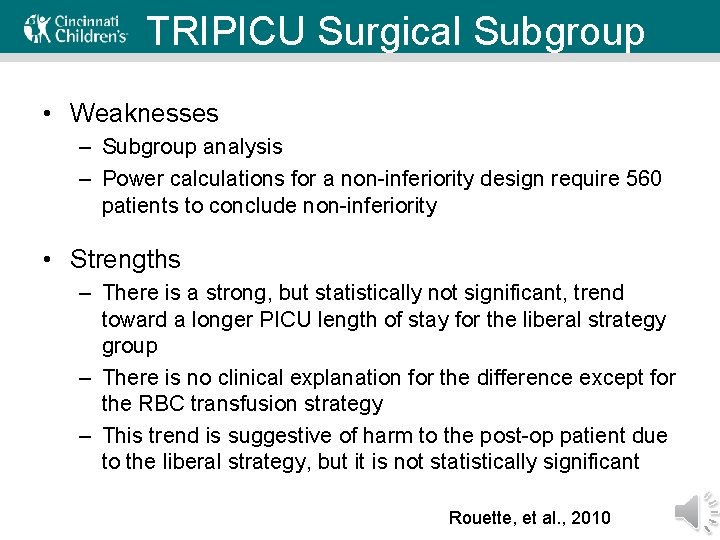
TRIPICU Surgical Subgroup • Weaknesses – Subgroup analysis – Power calculations for a non-inferiority design require 560 patients to conclude non-inferiority • Strengths – There is a strong, but statistically not significant, trend toward a longer PICU length of stay for the liberal strategy group – There is no clinical explanation for the difference except for the RBC transfusion strategy – This trend is suggestive of harm to the post-op patient due to the liberal strategy, but it is not statistically significant Rouette, et al. , 2010

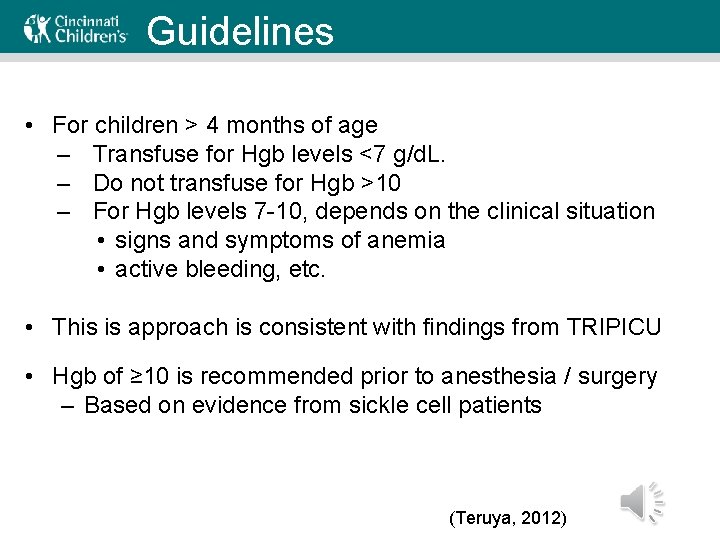
Guidelines • For children > 4 months of age – Transfuse for Hgb levels <7 g/d. L. – Do not transfuse for Hgb >10 – For Hgb levels 7 -10, depends on the clinical situation • signs and symptoms of anemia • active bleeding, etc. • This is approach is consistent with findings from TRIPICU • Hgb of ≥ 10 is recommended prior to anesthesia / surgery – Based on evidence from sickle cell patients (Teruya, 2012)

More Guidelines (Roseff et al. 2002)

Key Points • Understanding the physiology of oxygen delivery and how the body compensates for anemia is important in order to make decisions about giving blood transfusions. • Treating acute anemia with red blood cell transfusions comes with risks, which include transfusion reactions, fluid overload, and serious infections (e. g. Hep B). • The TRIPICU study suggests for asymptomatic children with acute anemia that it is safe to use a hemoglobin of <7 g/d. L as the red blood cell transfusion threshold and that there no benefits to transfusing if the hemoglobin is 7. 0 or greater.

Questions for Review • Consider the patient you were consulted on earlier. Her exam is normal, she is not actively bleeding. Her mom still thinks she would feel better if she weren’t so anemic. What are some of the possible complications of RBC transfusion? • Which are rare? Serious?

Further Discussion • Based on the TRIPICU study, are you comfortable not transfusing until Hgb < 7? – In which clinical situations would you want to transfuse before the Hgb falls below 7?

References 1. Anand N, Idio FG, Jr. , Remer S, Hoppenfeld S. The effects of perioperative blood salvage and autologous blood donation on transfusion requirements in scoliosis surgery. Journal of spinal disorders. Dec 1998; 11(6): 532 -534. 2. Carson JL, Noveck H, Berlin JA, Gould SA. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. Jul 2002; 42(7): 812 -818. 3. Copley LA, Richards BS, Safavi FZ, Newton PO. Hemodilution as a method to reduce transfusion requirements in adolescent spine fusion surgery. Spine. Feb 1 1999; 24(3): 219222; discussion 223 -214. 4. Crowell JW, Smith EE. Determinant of the optimal hematocrit. Journal of applied physiology. Mar 1967; 22(3): 501 -504. 5. Florentino-Pineda I, Blakemore LC, Thompson GH, Poe-Kochert C, Adler P, Tripi P. The Effect of epsilon-aminocaproic acid on perioperative blood loss in patients with idiopathic scoliosis undergoing posterior spinal fusion: a preliminary prospective study. Spine. May 15 2001; 26(10): 1147 -1151. 6. Franchini M, Gandini G, Regis D, De Gironcoli M, Cantini M, Aprili G. Recombinant human erythropoietin facilitates autologous blood collections in children undergoing corrective spinal surgery. Transfusion. Jul 2004; 44(7): 1122 -1124. 7. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. The New England journal of medicine. Feb 11 1999; 340(6): 409 -417. 8. Istaphanous GK, Wheeler DS, Lisco SJ, Shander A. Red blood cell transfusion in critically ill children: a narrative review. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. Mar 2011; 12(2): 174 -183.

9. Kleinman S, Carson JL. Indications for red cell transfusion in the adult. www. uptodate. com. January 15, 2013. 10. Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. The New England journal of medicine. Apr 19 2007; 356(16): 1609 -1619. 11. Morley SL. Red blood cell transfusions in acute paediatrics. Archives of disease in childhood. Education and practice edition. Jun 2009; 94(3): 65 -73. 12. Neilipovitz DT, Murto K, Hall L, Barrowman NJ, Splinter WM. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesthesia and analgesia. Jul 2001; 93(1): 82 -87. 13. Regis D, Franchini M, Corallo F, Bartolozzi P. Recombinant human erythropoietin in pediatric patients: efficacy in facilitating autologous blood donation in spinal deformity surgery. La Chirurgia degli organi di movimento. Oct-Dec 2004; 89(4): 299 -303. 14. Roseff SD, Luban NL, Manno CS. Guidelines for assessing appropriateness of pediatric transfusion. Transfusion. Nov 2002; 42(11): 1398 -1413. 15. Rouette J, Trottier H, Ducruet T, et al. Red blood cell transfusion threshold in postsurgical pediatric intensive care patients: a randomized clinical trial. Annals of surgery. Mar 2010; 251(3): 421 -427. 16. Sachs B, Delacy D, Green J, et al. Recombinant activated factor VII in spinal surgery: a multicenter, randomized, double-blind, placebo-controlled, dose-escalation trial. Spine. Oct 1 2007; 32(21): 2285 -2293. 17. Teruya J. Indications for red blood cell transfusion in infants and children. www. uptodate. com. November 27, 2012. 18. Teruya J. Administration and complications of red cell transfusion in infants and children. www. uptodate. com. January 16, 2013. 19. Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA : the journal of the American Medical Association. Jan 21 1998; 279(3): 217221.