POSTMARKETING ADVERSE DRUG EXPERIENCE INSPECTIONAL PROGRAM CDR Thomas





























- Slides: 31

POSTMARKETING ADVERSE DRUG EXPERIENCE INSPECTIONAL PROGRAM CDR Thomas R. Berry, RPh FDA, Investigator RAL-RP / ATL-DO

PADE Inspectional Program § Headquarters scientists use adverse event reports to evaluate the safety of marketed drugs § OC/ADE Team coordinates between regulatory, investigational, and scientific staff § Field investigators assure industry compliance with reporting regulations

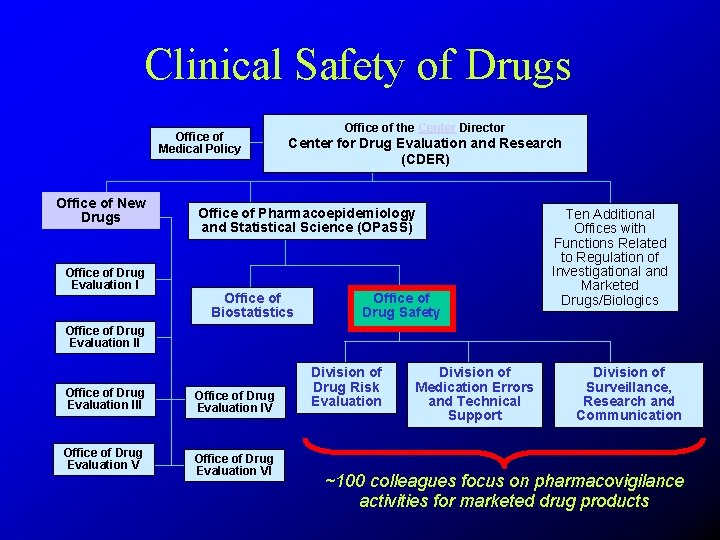
Clinical Safety of Drugs Office of Medical Policy Office of New Drugs Office of Drug Evaluation I Office of the Center Director Center for Drug Evaluation and Research (CDER) Office of Pharmacoepidemiology and Statistical Science (OPa. SS) Office of Biostatistics Office of Drug Safety Ten Additional Offices with Functions Related to Regulation of Investigational and Marketed Drugs/Biologics Office of Drug Evaluation III Office of Drug Evaluation V Office of Drug Evaluation IV Office of Drug Evaluation VI Division of Drug Risk Evaluation Division of Medication Errors and Technical Support Division of Surveillance, Research and Communication ~100 colleagues focus on pharmacovigilance activities for marketed drug products

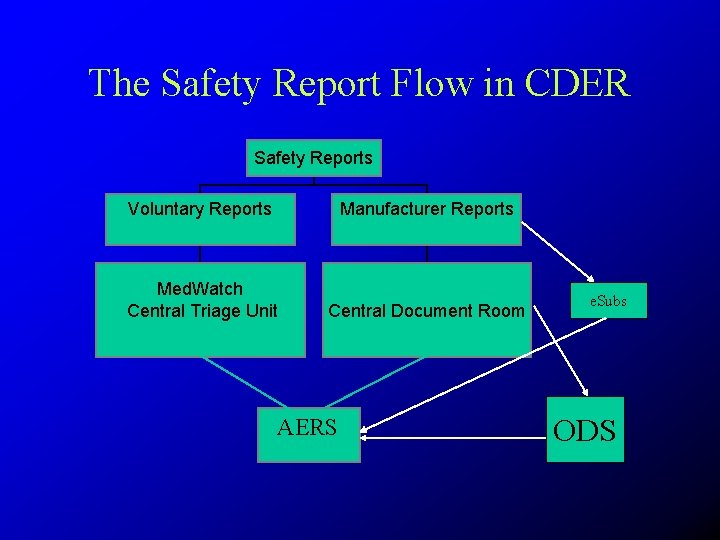
The Safety Report Flow in CDER Safety Reports Voluntary Reports Manufacturer Reports Med. Watch Central Triage Unit Central Document Room AERS e. Subs ODS

FDA Adverse Event Reporting System Database • Database Origin 1969 • 3 million reports in database • Contains Drug , Therapeutic Biologic and Therapeutic Blood Reports • exception = vaccines VAERS 1 -800 -822 -7967

Source of Individual Safety Reports for AERS Reports from manufacturers to FDA > 90% of all reports FDA database “AERS” Reports to FDA directly from individuals <10% of all reports (“direct”)

Purpose of ADE Regulations § To obtain additional information on adverse events that may not have been detected prior to marketing § To improve the labeling of drug products

Reporting Regulations § 21 CFR Sections § 310. 305 – Rx drugs not subject to approved applications “Grandfathered” drugs that were marketed pre-1938 § 314. 80 – Rx drugs subject to NDAs/ANDAs and OTCs associated with approved applications (Marketed Drugs) § 314. 98 – Rx drugs subject to ANDAs (Generic Drugs) § Compliance Program Guideline 7353. 001 09/30/99 § Guidance for Industry – Postmarketing Safety Reporting for Human Drug and Biological Products including Vaccines March 2001

Adverse Drug Experience § Any adverse event associated with the use of drugs in humans whether or not it is considered drug related

Serious Adverse Drug Experience § § § Death Life threatening (per initial reporter) Permanently or significantly disabling Hospitalization Congenital anomaly / birth defect Important medical event

Unexpected Adverse Drug Event § Not listed in current labeling § Listed in labeling but greater specificity or severity renal impairment listed, patient experiences renal failure

Reporting Requirements § Within 15 calendar days if serious and unexpected (domestic and foreign) § Follow-up information § Non-applicant notifies applicant within five calendar days

Regulatory Start Clock § First day a firm or any affiliate receives event data containing all four elements: § § An identifiable patient An identifiable reporter A suspect drug An adverse event or fatal outcome

Reporting Forms § 3500 A (Med. Watch Form) § Council for International Organization of Medical Science (CIOMS I Foreign) § Other form if approved by FDA in advance



Literature Searches § The 15 -day reporting requirements apply only to reports found in scientific and medical journals either as case reports or as the result of a formal clinical trial. § Form 3500 A (Med. Watch) § Copy of the published article

Periodic Reports § Quarterly and Annual Reports § Quarterly for the first 3 years, then annually § Usually based on the date the NDA/ANDA was approved § Serious and Expected ADEs § All Non-serious ADEs

Recordkeeping § Maintain for 10 years - Records of all adverse drug experiences § Raw Data § Any correspondence relating to adverse drug experiences

Role of the Investigator § To verify through on-site visits that firms are submitting all required reports of adverse events to FDA and the reports are complete, accurate and timely § Document violations to support appropriate regulatory action

We are looking for … § Outcomes to identify problem areas for inspectional coverage § What system failure(s) caused the firm not to comply with the regulations § Patterns and trends

Source Documents § SOPs – should specify control for all functions and duties for the surveillance, receipt, evaluation and reporting of ADEs to the FDA. § Call log § Complaint logs § Contracts for PADE services § Training

Note Discrepancies § § § Omissions Minimizing results Lack of Drug Effectiveness Potential Product Complaints Lack of follow-up Inadequate follow-up

If reports are found … § Determine the nature of the event and the cause for failure to report § Determine what changes have been made to prevent reoccurrence § Late 15 day alert reports - Explanation in Med. Watch narrative (Recommended) - Explanation in transmittal letter (Recommended)

Types of Violations § Serious and unlabeled events not submitted in a timely manner (range 30 days up to 10 years) § Lack of assurance that all ADEs were submitted § Foreign serious and unlabeled events not submitted from foreign affiliates

Types of Violations § Failure to conduct prompt and adequate follow -up investigations of ADEs § Serious and unlabeled events not assessed properly § SOPs are not established for the surveillance, receipt, evaluation, and reporting to FDA of postmarketing adverse drug experiences § No periodic reports submitted

Current Issues § HEALTH INSURANCE PORTABILITY AND ACCOUNTABILITY ACT (HIPAA) § Transfer of responsibility for 21 CFR 314. 80 from the applicant to affiliates / contractors

Turbo EIR § Provides more consistency between inspections and investigators § Provides specific citations and examples of violations

Internet Sites for ADR Information § www. fda. gov/cder/aers/default. htm § www. fda. gov/medwatch § http: //www. fda. gov/cder/learn/ADE_Pagex. htm

PADE Compliance Team CDER Office of Compliance Denis Mackey mackey@cder. fda. gov (301 -827 -8926) Carol Krueger kruegerc@cder. fda. gov (301 -827 -8989)

PADE Inspectional Program Questions ? ? ?