Post cardiac arrest care SNUBH Joonghee Kim Overview

Post cardiac arrest care SNUBH Joonghee Kim

Overview

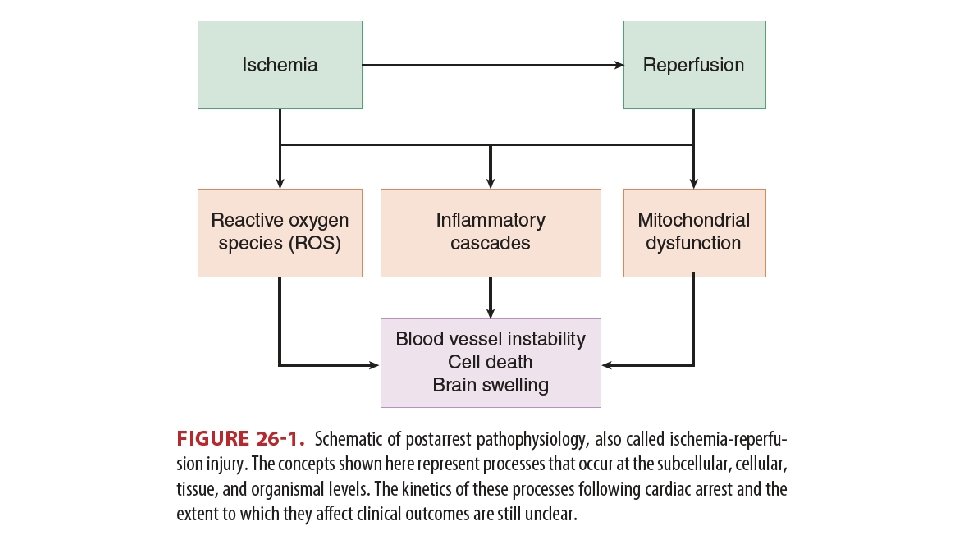
Pathophysiology • Persistent precipitating pathology • Cause of arrest • Anoxic brain injury • Can be aggravated by additional insults (fever, glucose, hypo/hyperoxia) • Post-cardiac arrest myocardial dysfunction • Up to several days • Systemic ischemia/reperfusion response • Inflammation, endothelial activation • Activation of immunologic and coagulation pathways • Sepsis-like syndrome Pothiawala, 2017

Cardiovascular care • 12 -lead ECG ASAP after ROSC • Prevalence of lesions requiring emergency treatment in cardiac-cause • in 96% of STEMI • in 58% without ST elevation
Recommendations • Emergent CAG in STEMI • Comatose, suspected cardiac, but without ST elevation • In select cases: e. g. unstable patients (electrically / hemodynamically) • Any indications for CAG • Irrespective of mental status • Considerations for selecting patients • i. e. stability, comorbidities, ongoing ischemia • Need to be separated from assessment of neurologic prognosis
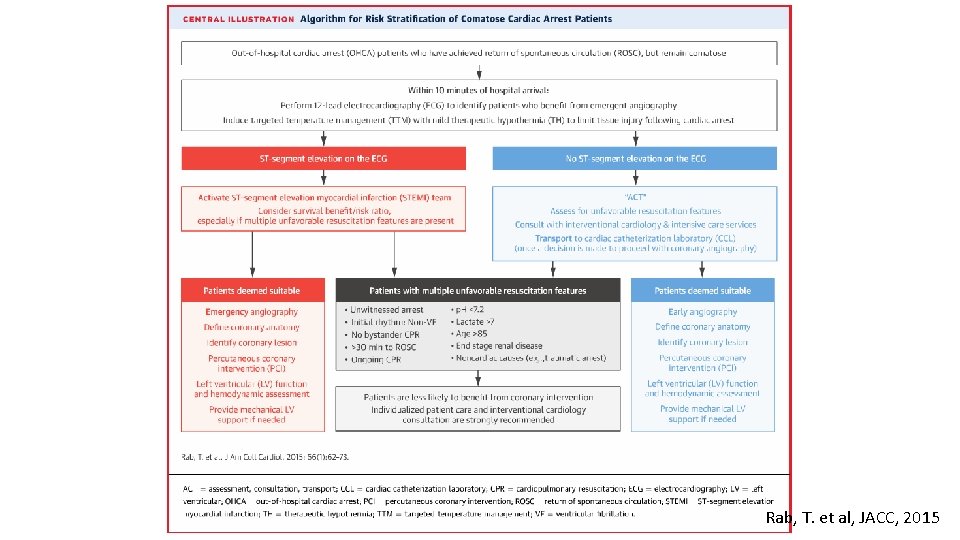
Rab, T. et al, JACC, 2015
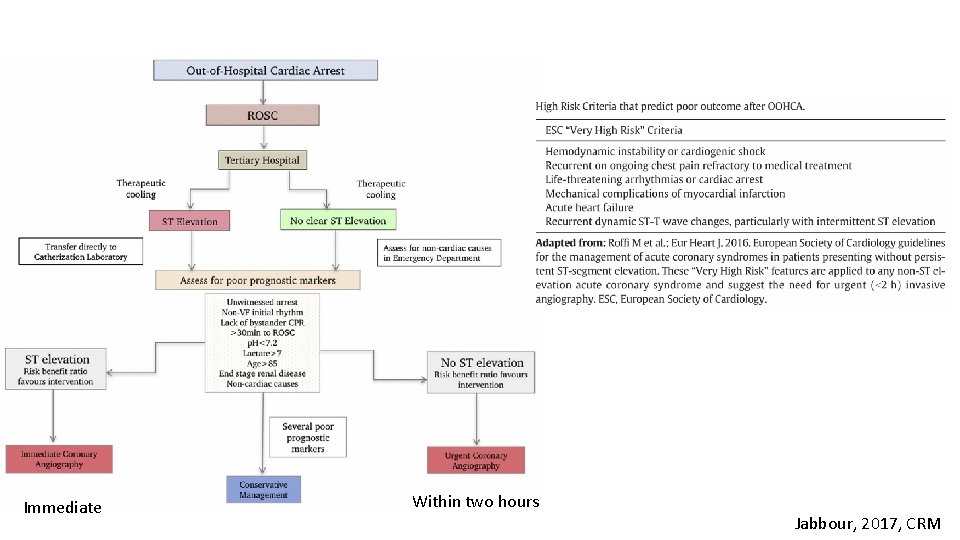
Immediate Within two hours Jabbour, 2017, CRM

Hemodynamic goals • Optimal goals are not defined • Observational studies: • SBP threshold • MBP threshold • Interventional studies: • Before and after • bundled care with BP goals • Impossible to separate the effects
Recommendations • True optimal BP will be different by patients and organs • SBP: 90 • MAP: 65 • Targets for other measures are not defined

Epinephrine • 0. 1– 0. 5 mcg/kg/min (In 70 -kg adult, 7– 35 mcg/min) • Useful for symptomatic bradycardia if atropine and transcutaneous pacing fail or if pacing is not available • Used to treat severe hypotension (eg, systolic blood pressure <70 mm Hg) • Useful for anaphylaxis associated with hemodynamic instability or respiratory distress

Norepinephrine • 0. 1– 0. 5 mcg/kg/min (In 70 -kg adult, 7– 35 mcg/min) • Used to treat severe hypotension (eg, systolic blood pressure <70 mm Hg) and a low total peripheral resistance • Relatively contraindicated in patients with hypovolemia. It may increase myocardial oxygen requirements, mandating cautious use in patients with ischemic heart disease • Usually induces renal and mesenteric vasoconstriction; in sepsis, however, norepinephrine improves renal blood flow and urine output

Dopamine • 5– 10 mcg/kg/min • Used to treat hypotension, especially if it is associated with symptomatic bradycardia • Although low-dose dopamine infusion has frequently been recommended to maintain renal blood flow or improve renal function, more recent data have failed to show a beneficial effect from such therapy
Dobutamine • 5– 10 mcg/kg/min • The (+) isomer is a potent beta-adrenergic agonist, whereas the (–) isomer is a potent alpha-1 -agonist • The vasodilating beta 2 -adrenergic effects of the (+) isomer counterbalance the vasoconstricting alpha-adrenergic effects, often leading to little change or a reduction in systemic vascular resistance

TTM • VF/p. VT: supported by RCTs • Non-shockable: unknown • In-hospital cardiac arrest: unknown • There are essentially no patients for whom temperature control somewhere in the range between 32 -36 is contraindicated.
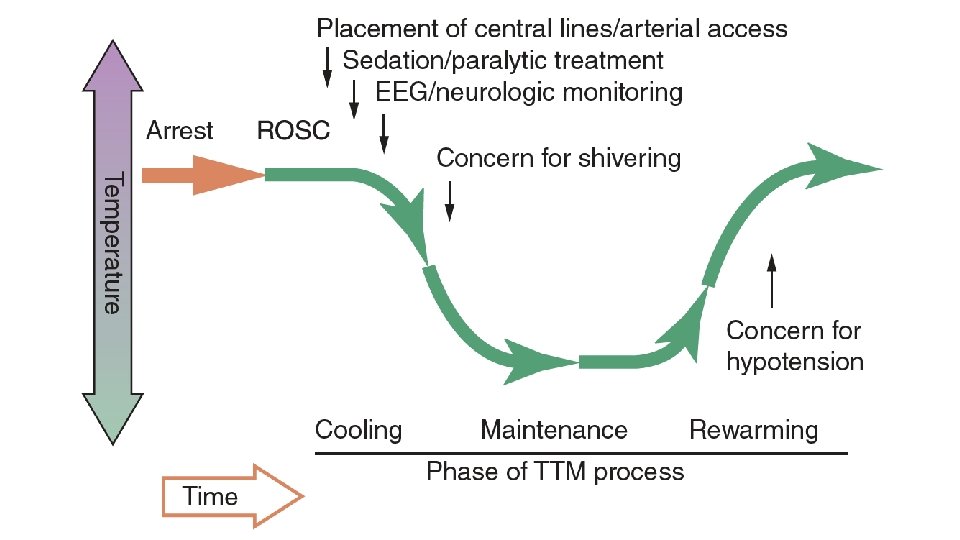
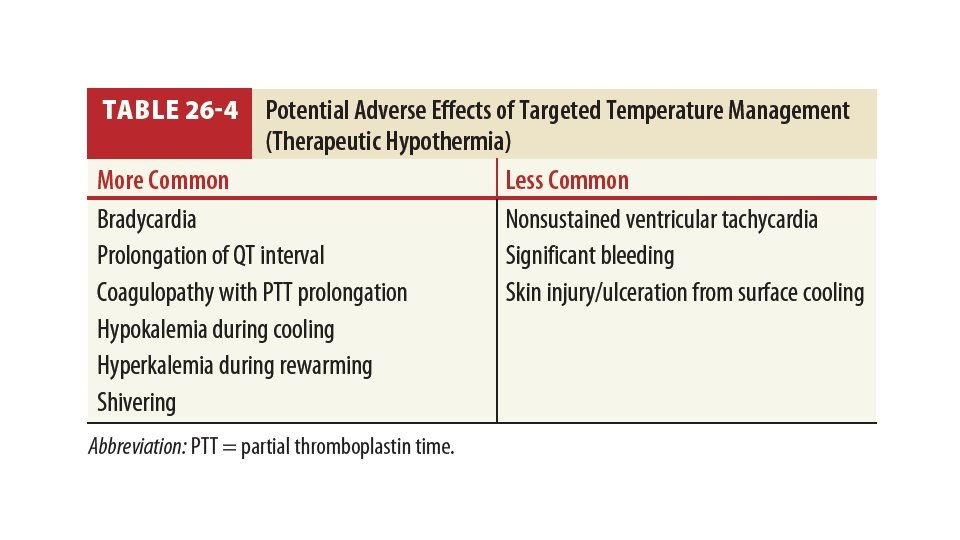
Recommendations • TTM on regardless of initial rhythm • Rarity of adverse effects • High neurologic morbidity / mortality • Temp. range: • 32 – 36 • At least 24 hours after achieving target temp. • Upper limit of duration is unknown • Temp sensitivity may continue as long as brain dysfunction continues

Temperature target • Higher target temp. • Bleeding • Higher initial temp. • Lower target temp. • Seizures / cerebral edema • Lower initial temp. – rapid / active warming is not suggested
Prehospital hypothermia • No clinical improvements (survival, neurologic outcomes) • Increased frequency of pulmonary edema after 2 L of cold saline • Recommendations • Against the routine prehospital cooling with rapid cold saline

Avoidance of hyperthermia • Fever after TTM • Fever after ROSC is associated with poor outcome
Recommendations • Active prevention after TTM (Expert opinion) • Though evidences are weak, • the intervention is relatively benign, • and association with worsened outcomes in other conditions • Simplest method continuing TTM

Other cares
Seizure management • Prophylactic administration – not helpful • No evidence to support a specific drug or combination • Recommendations • Should be monitored frequently or continuously in comatose patients • Same AEDs used in status epilepticus caused by other etiologies

Respiratory care • Hypocapnia – associated with worse outcomes • Recommendations • Normocarbia (Et. CO 2 30 -40 mm. Hg, Pa. CO 2 35 -45 mm. Hg) • 2010: 35 -40, 40 -45 while avoiding hemodynamic compromise • Individualized approach • Acute lung injury – higher Pa. CO 2 • Cerebral edema – lower Pa. CO 2 • Hyperventilation-induced vasoconstriction vs. correction of metabolic acidosis

Oxygenation • Conventional definition • Hypoxia: Pa. O 2 < 60 mm. Hg • Hyperoxia: Pa. O 2 > 300 mm. Hg • Prevention of hypoxic episodes is more important

Recommendations • After ROSC, • Maximal O 2 concentration until titration is possible • Lower Fi. O 2 if Sat. is 100% • If sat. can be maintained at 94% or greater.

Pulmonary embolism • Recommendation (2010) • In post–cardiac arrest patients with arrest due to presumed or known pulmonary embolism, fibrinolytics may be considered.

Sedation • Recommendation (2010) • It is reasonable to consider the titrated use of sedation and analgesia in critically ill patients who require mechanical ventilation or shivering suppression during induced hypothermia after cardiac arrest.

Glucose control - Recommendation •

Prognostication

Prognostication • Accurate prediction of poor outcome • FPR close to 0% with narrow 95% confidence interval

Cerebral performance category (CPC) score • CPC 1: full recovery • CPC 2: moderate disability (independent daily activity) • CPC 3: severe neurological disability but preserved consciousness • CPC 4: coma or persistent vegetative state • CPC 5: death

Why prognostication is difficult? • Heterogeneity of patient population • Poorly described evaluation procedures • Self-fulfilling prophecy • Poor outcome measure scale • TTM
Heterogeneity of patient population • Original meaning of coma: complete unresponsiveness • Most studies • Do not specify what is meant by ‘comatose’ • Do not use specific Glasgow Coma Scale (GCS) cut-off • Common use • “meaningful response to verbal commands” • “unable to follow verbal commands or show purposeful movement”

Poorly described evaluation procedures • Sedated/paralyzed? • Sufficient stimulus? • Electrophysiological testing - technically challenging. • Significant laboratory variations • Recent advancement of neuroimaging - comparisons between studies inherently questionable.

Self-fulfilling prophecy • Withdrawal of life-sustaining therapies (WLST) A major limitation • 2006 PROPAC study - high WLST • • 407 patients at 32 Dutch hospitals SSEP test showed very low FPR WLST in 23% at 24 h & 28% at 48 h after ROSC Absence of SSEPs at 72 h - considered as a sufficiently reliable predictor of poor outcome • Cronberg et al. - a minimum of 72 h post rewarming or 4. 5 days postarrest.

Poor outcome measure scale • Crude measurement • Neurologic impairment • Psychological impairment • Ability to return to work • Mixed concept • Mortality • Neurologic function • Heterogeneity among studies
Therapeutic hypothermia • Use of paralytics and sedatives • NMB • Motor response, corneal reflex, eye movement impaired • PLR and pupil size preserved, but influenced by other medications • Impaired drug metabolism • increased fentanyl, midazolam and propofol concentrations • Altered electrophysiological tests (particularly EEG) • Delayed neurological recovery
Timing of prediction • When FPRs approach 0% • TTM Slower drug metabolization Residual sedation/paralysis • Minimum of 72 hours after ROSC without TTM • Some period after rewarming with TTM
Recommendations • Clinical examination • With TTM: 72 hours after return of normothermia • Operationally 4. 5 -5 days after ROSC • Without TTM: 72 hours after cardiac arrest • Can be longer with residual sedative/paralytic effect

Clinical examination findings • PLR • generally not inhibited by NMB • Corneal reflex • inhibited by NMB • Motor response • inhibited by NMB
Recommendations • No PLR at 72 hours • Without TTM: FPR 0% • With TTM: FPR 1% • No motor response or extensor posturing • Should not be used alone • Maybe, to identify the candidates for prognostication • Myoclonus • Should not be used – FPR 5% • Status myoclonus during 72 -120 hours after cardiac arrest • In combination with other tests

EEG and treatment of seizure • Cortical activity • Seizures • Lack of standardized EEG terminology

Predictors in EEG • Burst suppression • After rewarming - 0% FPR • Epileptiform activity • But not with reactive EEG • No reactivity • After rewarming - 0% FPR • Low voltage EEG, low BIS / EEG grade not reliable
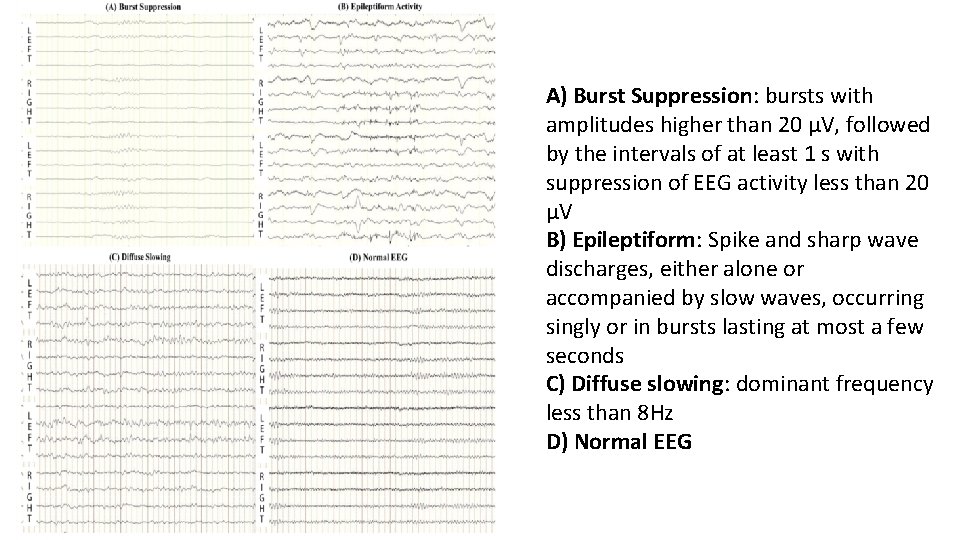
A) Burst Suppression: bursts with amplitudes higher than 20 μV, followed by the intervals of at least 1 s with suppression of EEG activity less than 20 μV B) Epileptiform: Spike and sharp wave discharges, either alone or accompanied by slow waves, occurring singly or in bursts lasting at most a few seconds C) Diffuse slowing: dominant frequency less than 8 Hz D) Normal EEG
Recommendations • With TTM • Absence of EEG reactivity at 72 hours after cardiac arrest • Persistent burst suppression on EEG after rewarming • Intractable and persistent status epilepticus without EEG reactivity ( 72 or more) • Without TTM • Presence of burst suppression on EEG at 72 hours in combination with other predictors
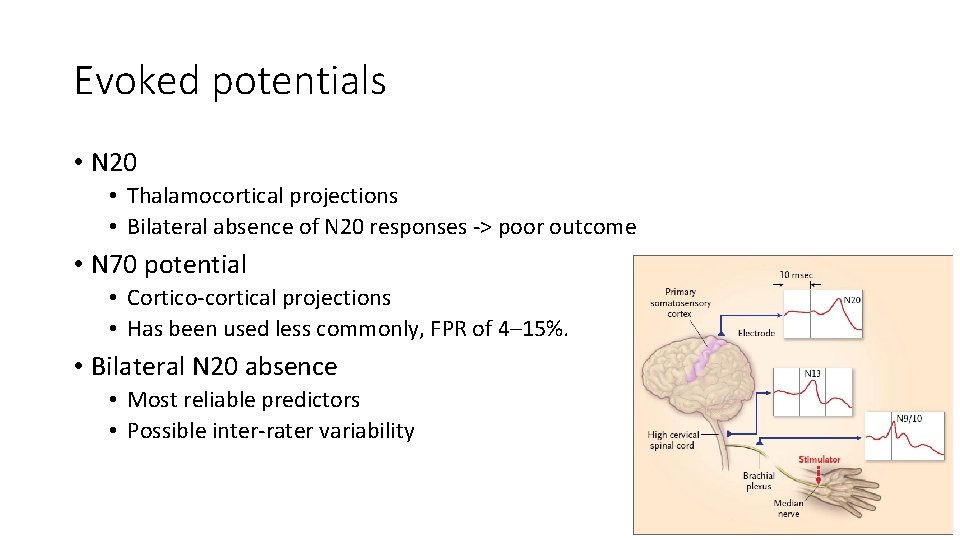
Evoked potentials • N 20 • Thalamocortical projections • Bilateral absence of N 20 responses -> poor outcome • N 70 potential • Cortico-cortical projections • Has been used less commonly, FPR of 4– 15%. • Bilateral N 20 absence • Most reliable predictors • Possible inter-rater variability

Evoked potentials • Has been used as the parameter for withdrawal of life-sustaining therapy self-fulfilling prophecy • Recommendations • Bilateral absence of N 20 24 -72 hours after cardiac arrest or rewarming

Imaging tests • CT • SAH • Brain edema – decreased gray-white ratio • MRI • DWI, quantification using ADC values
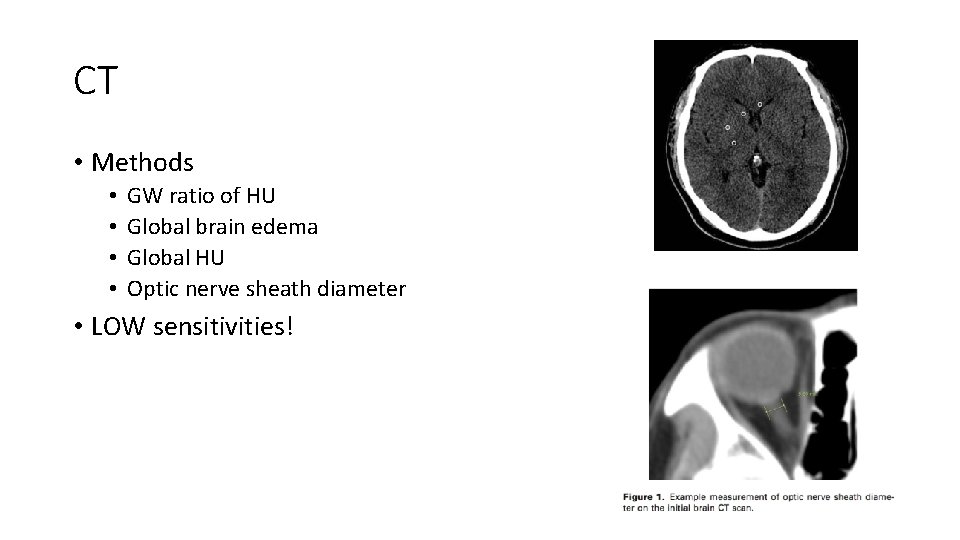
CT • Methods • • GW ratio of HU Global brain edema Global HU Optic nerve sheath diameter • LOW sensitivities!
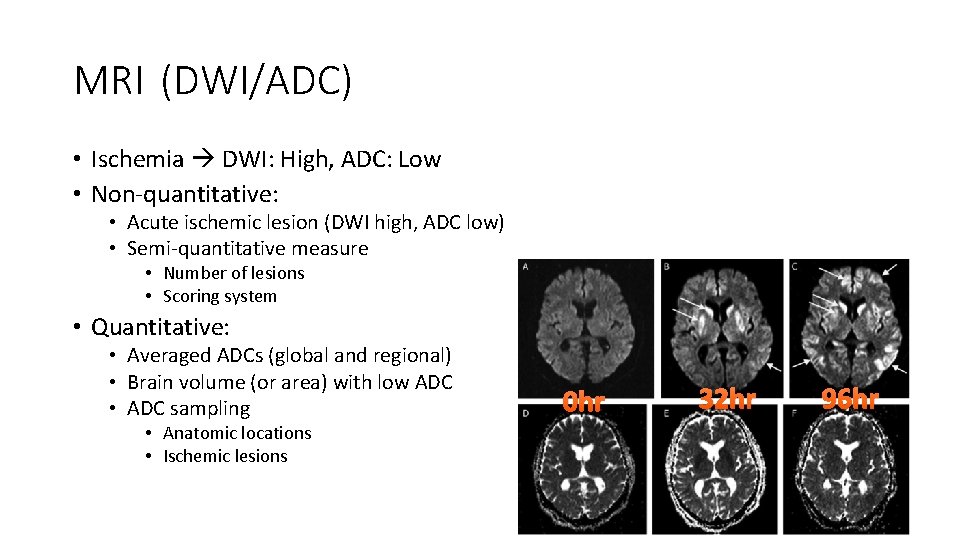
MRI (DWI/ADC) • Ischemia DWI: High, ADC: Low • Non-quantitative: • Acute ischemic lesion (DWI high, ADC low) • Semi-quantitative measure • Number of lesions • Scoring system • Quantitative: • Averaged ADCs (global and regional) • Brain volume (or area) with low ADC • ADC sampling • Anatomic locations • Ischemic lesions 0 hr 32 hr 96 hr
Recommendations • Reasonable to use the presence of marked reduction of the GWR on brain CT obtained within 2 hours after cardiac arrest • Extensive restriction of diffusion on brain MRI at 2 -6 days after cardiac arrest in combination with other established predictors • Not fully standardized and are subject to interobserver variability

Blood markers • NSE, S-100 B • No reliable threshold values • Not specific to neuronal damages • Hemolysis, neuroendocrine tumors, myenteric plexus, muscle, adipose tissue breakdown

Recommendations • Should not be used alone • With other tests, at 72 hours or more after cardiac arrest • Especially if repeated samplings are consistent
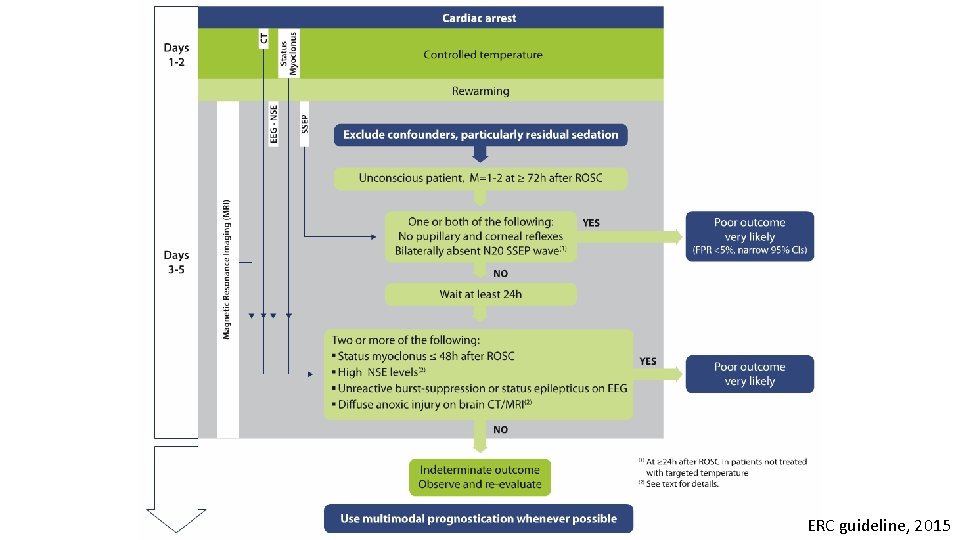
ERC guideline, 2015

Summaries
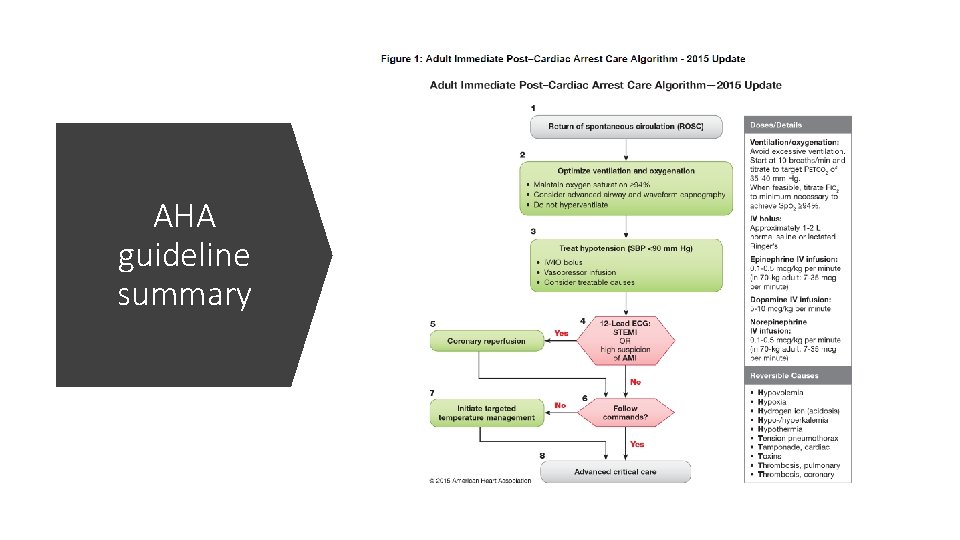
AHA guideline summary
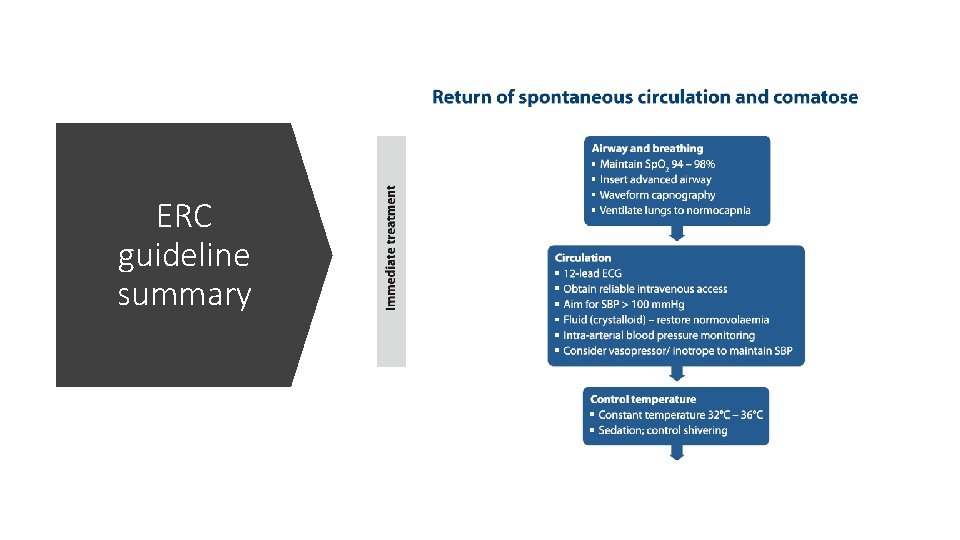
ERC guideline summary
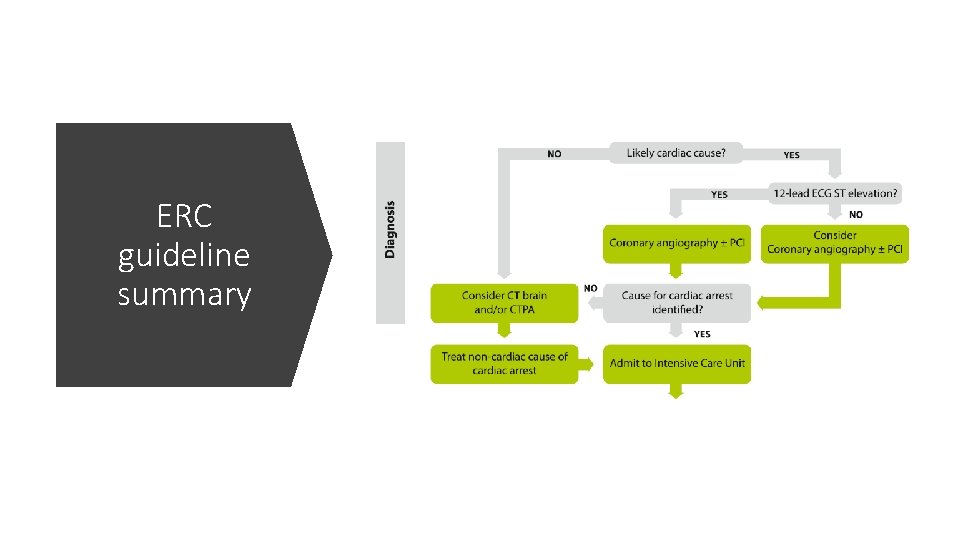
ERC guideline summary
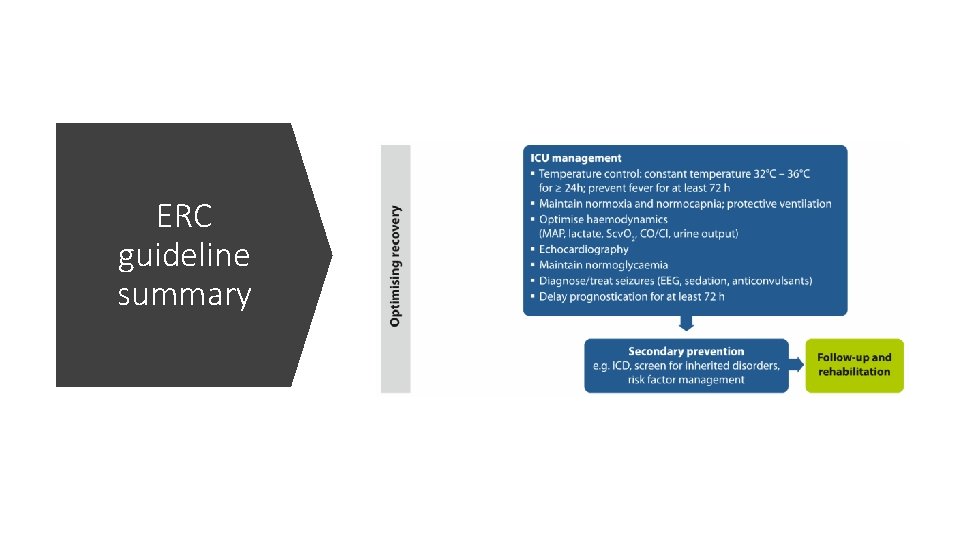
ERC guideline summary







문 2013> 심정지 환자의 자발순환 회복 이후 생리적 지표로 적절 한 것은? ① SBP 80 mm. Hg ② MAP 60 mm. Hg ③ Sp. O 2 96% ④ Pa. CO 2 35 mm. Hg ⑤ ETCO 2 20 mm. Hg • Normocarbia (Et. CO 2 30 -40 mm. Hg, Pa. CO 2 35 -45 mm. Hg) • 2010: 35 -40, 40 -45 while avoiding hemodynamic compromise







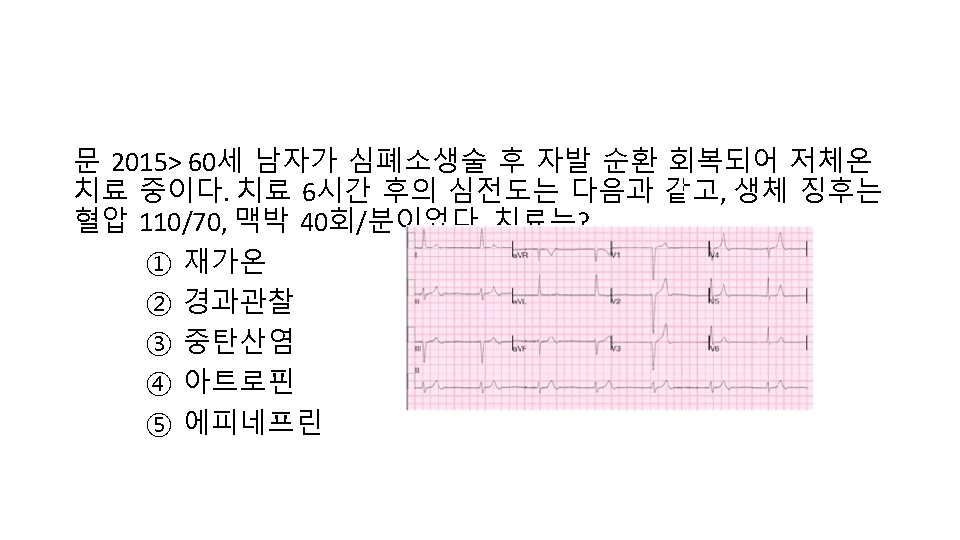
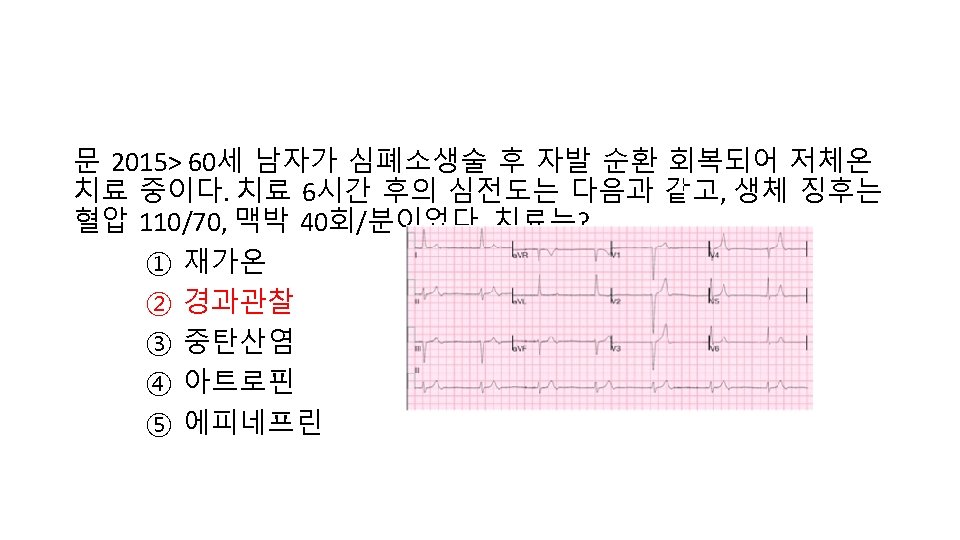




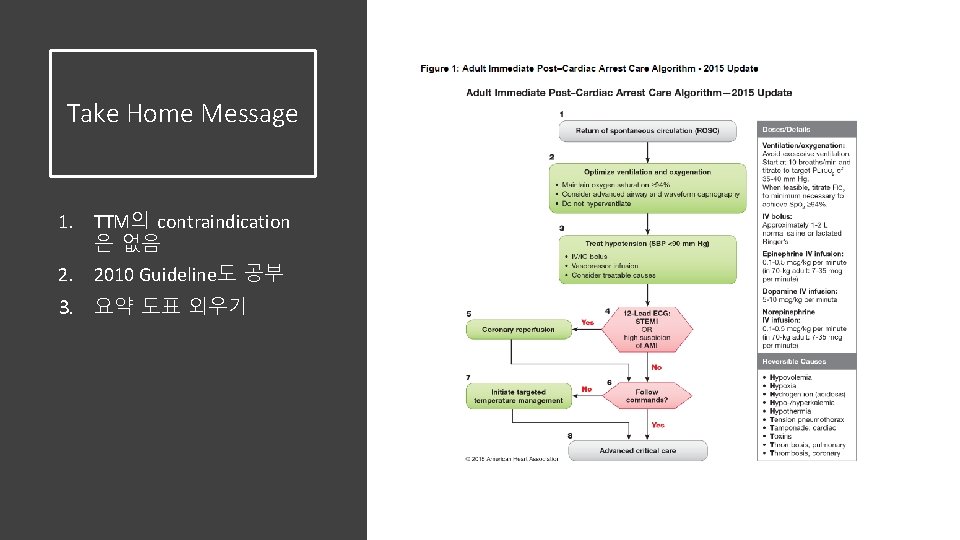
Take Home Message 1. TTM의 contraindication 은 없음 2. 2010 Guideline도 공부 3. 요약 도표 외우기
- Slides: 83