POSITION OF HYDROGEN IN THE PERIODIC TABLE DIHYDROGEN







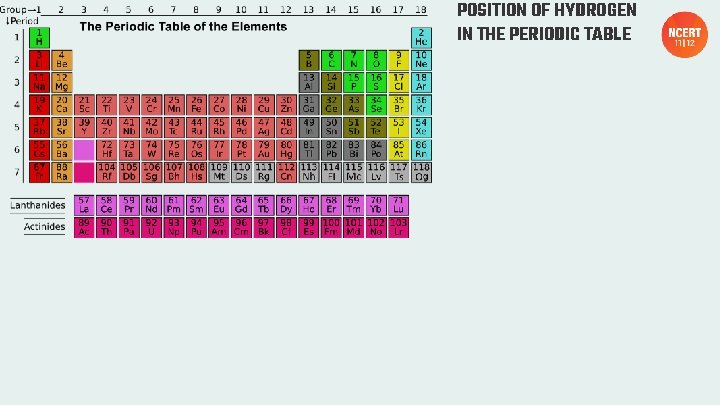
POSITION OF HYDROGEN IN THE PERIODIC TABLE

DIHYDROGEN, 2 H ● Most abundant element in the universe ● The combined form it constitutes 15. 4% of the earth's crust and the oceans.
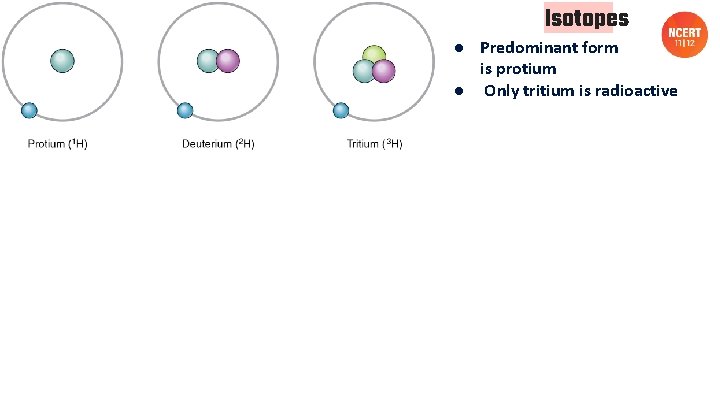
Isotopes ● Predominant form is protium ● Only tritium is radioactive

HYDRIDES Ionic or saline or salt like hydrides Covalent or molecular hydrides Metallic or nonstoichiometric hydrides

Ionic or saline or salt like hydrides
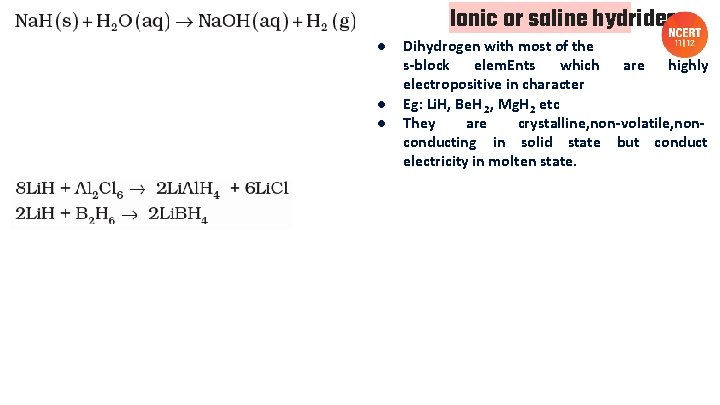
● ● ● Ionic or saline hydrides Dihydrogen with most of the s-block elem. Ents which are highly electropositive in character Eg: Li. H, Be. H 2, Mg. H 2 etc They are crystalline, non-volatile, nonconducting in solid state but conduct electricity in molten state.
Covalent or molecular hydrides
Covalent or Molecular hydrides ● Dihydrogen with p block elements ● They are further classified into three types- electron deficient, electron precise, electron-rich

Metallic or nonstoichiometric hydrides
Metallic or non stoichiometric hydrides ● ● d-block and f-block elements Group 7, 8 and 9 metals do not form hydride. The absorption of hydrogen on transition metals is used in catalytic reduction / hydrogenation reactions Pd and Pt act as Hydrogen storage and energy source

Water

Physical properties of water ● ● High freezing point, high boiling point, high heat of vaporisation and high heat of fusion in comparison to H 2 S and H 2 Se Higher specific heat, thermal conductivity, surface tension, dipole moment and dielectric constant
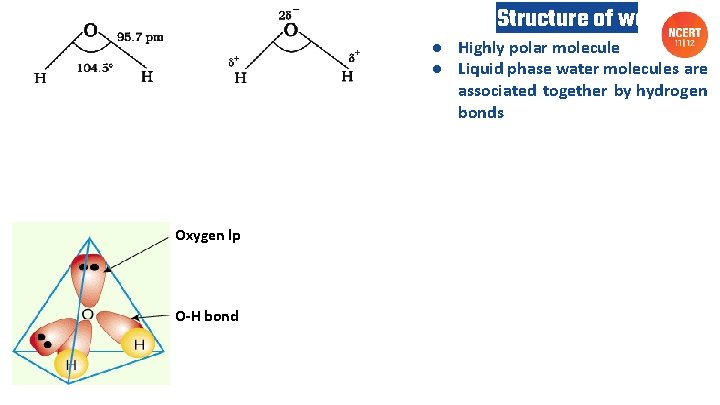
Structure of water ● Highly polar molecule ● Liquid phase water molecules are associated together by hydrogen bonds Oxygen lp O-H bond
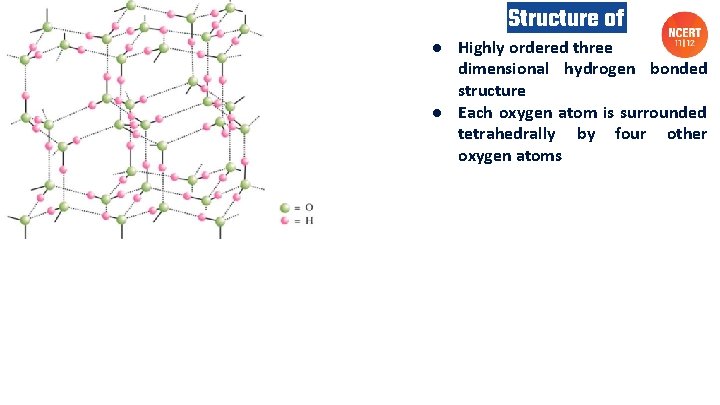
Structure of ice ● Highly ordered three dimensional hydrogen bonded structure ● Each oxygen atom is surrounded tetrahedrally by four other oxygen atoms

Chemical Properties of water

Amphoteric Nature ● Act as an acid as well as a base ● Autoprotolysis (self-ionization) water of
Redox Reactions Involving Water ● Easily reduced to dihydrogen by highly electropositive metals ● With fluorine it is oxidised to oxygen.

Hydrolysis Reaction ● Strong hydrating tendency and dissolves many ionic compounds. ● Many covalent compounds are hydrolysed.

Hydrates formation ● From aqueous solutions many salts can be crystallised as hydrated salts

Hydrates formation

Hard and soft water Soft Water Hard water
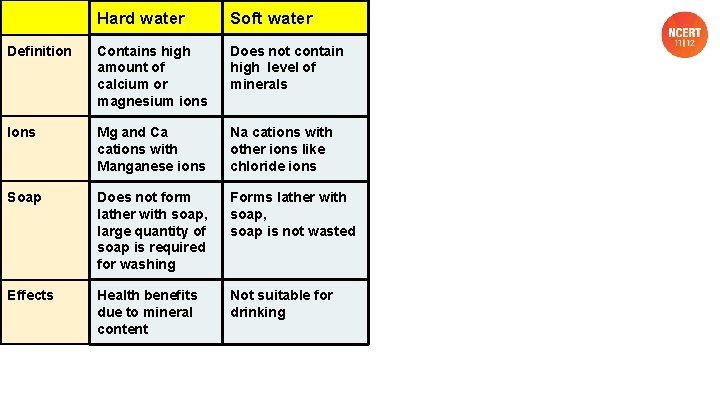
Hard water Soft water Definition Contains high amount of calcium or magnesium ions Does not contain high level of minerals Ions Mg and Ca cations with Manganese ions Na cations with other ions like chloride ions Soap Does not form lather with soap, large quantity of soap is required for washing Forms lather with soap, soap is not wasted Effects Health benefits due to mineral content Not suitable for drinking
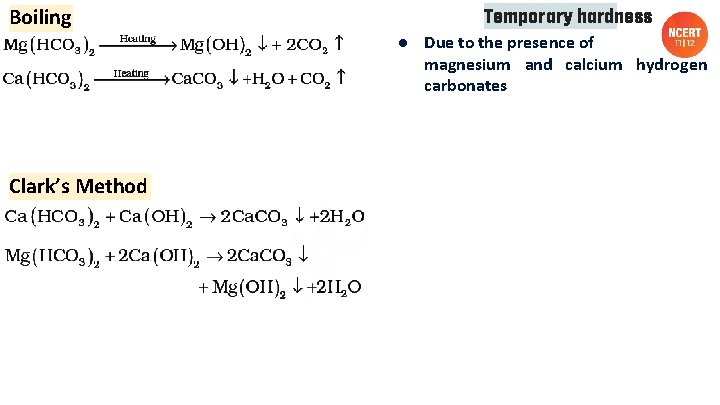
Boiling Temporary hardness ● Due to the presence of magnesium and calcium hydrogen carbonates Clark’s Method
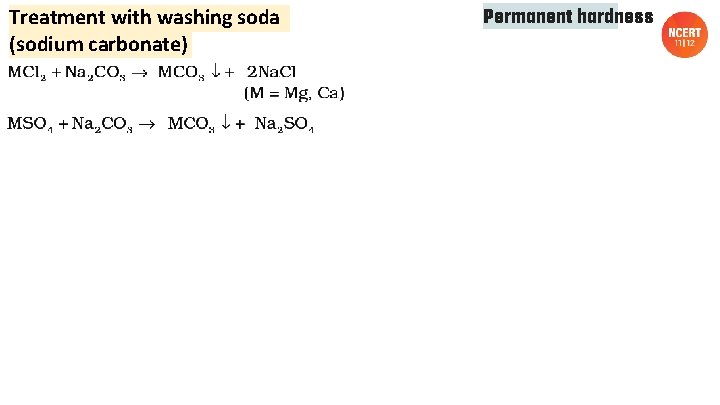
Treatment with washing soda (sodium carbonate) Permanent hardness
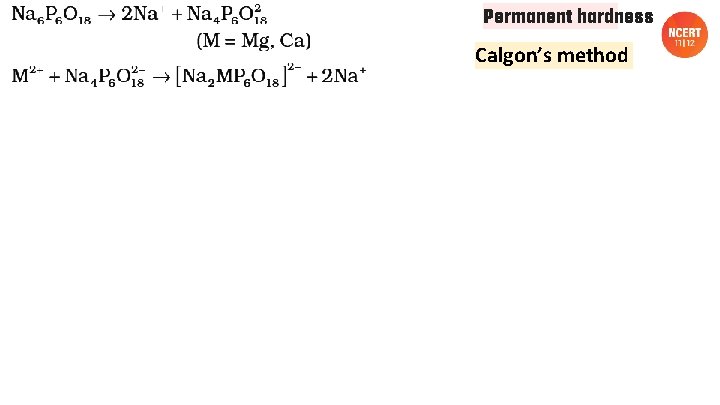
Permanent hardness Calgon’s method

Permanent hardness Ion-exchange method zeolite/permutit process

Permanent hardness Synthetic resins method
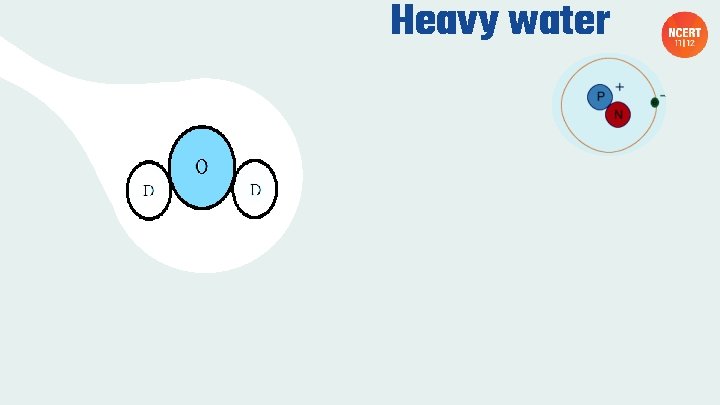
Heavy water
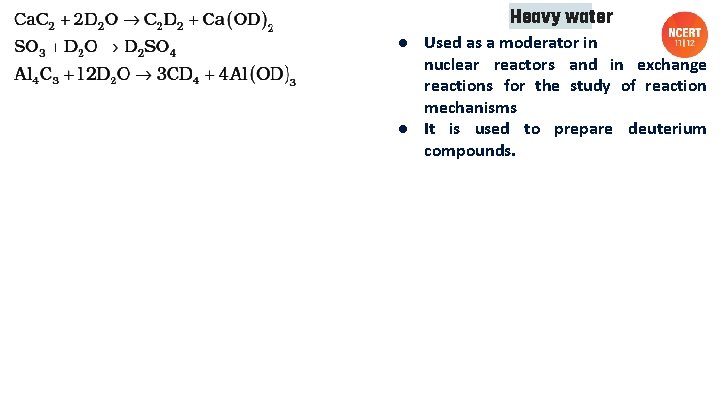
Heavy water ● Used as a moderator in nuclear reactors and in exchange reactions for the study of reaction mechanisms ● It is used to prepare deuterium compounds.
Dihydrogen as a fuel
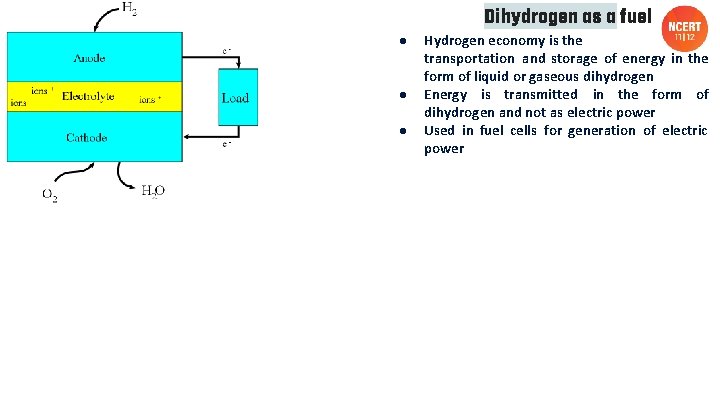
● ● ● Dihydrogen as a fuel Hydrogen economy is the transportation and storage of energy in the form of liquid or gaseous dihydrogen Energy is transmitted in the form of dihydrogen and not as electric power Used in fuel cells for generation of electric power


Class 11 Cass 12 2 PM 2 PM 2 PM Mon Tue Wed Thu Fri Sat



THANKS
- Slides: 45