Population Pharmacokinetics and ExposureResponse Modeling and Simulation to
- Slides: 1

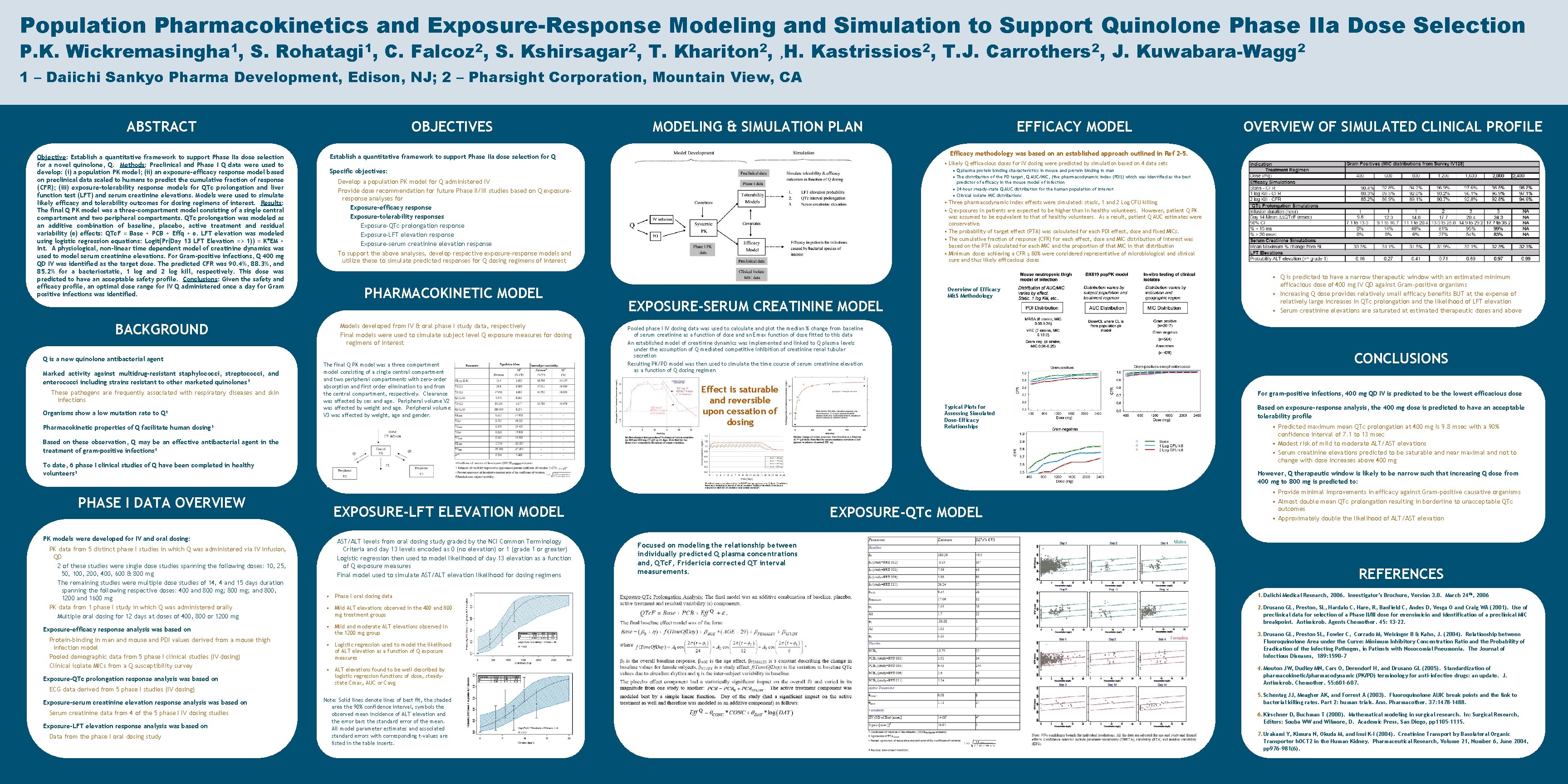
Population Pharmacokinetics and Exposure-Response Modeling and Simulation to Support Quinolone Phase IIa Dose Selection P. K. Wickremasingha 1, S. Rohatagi 1, C. Falcoz 2, S. Kshirsagar 2, T. Khariton 2, , H. Kastrissios 2, T. J. Carrothers 2, J. Kuwabara-Wagg 2 1 – Daiichi Sankyo Pharma Development, Edison, NJ; 2 – Pharsight Corporation, Mountain View, CA ABSTRACT Objective: Establish a quantitative framework to support Phase IIa dose selection for a novel quinolone, Q. Methods: Preclinical and Phase I Q data were used to develop: (i) a population PK model; (ii) an exposure-efficacy response model based on preclinical data scaled to humans to predict the cumulative fraction of response (CFR); (iii) exposure-tolerability response models for QTc prolongation and liver function test (LFT) and serum creatinine elevations. Models were used to simulate likely efficacy and tolerability outcomes for dosing regimens of interest. Results: The final Q PK model was a three-compartment model consisting of a single central compartment and two peripheral compartments. QTc prolongation was modeled as an additive combination of baseline, placebo, active treatment and residual variability (ε) effects: QTc. F = Base + PCB + Effq + ε. LFT elevation was modeled using logistic regression equations: Logit(Pr(Day 13 LFT Elevation => 1)) = K*EM + Int. A physiological, non-linear time dependent model of creatinine dynamics was used to model serum creatinine elevations. For Gram-positive infections, Q 400 mg QD IV was identified as the target dose. The predicted CFR was 90. 4%, 88. 3%, and 85. 2% for a bacteriostatic, 1 log and 2 log kill, respectively. This dose was predicted to have an acceptable safety profile. Conclusions: Given the safety and efficacy profile, an optimal dose range for IV Q administered once a day for Gram positive infections was identified. BACKGROUND Q is a new quinolone antibacterial agent Marked activity against multidrug-resistant staphylococci, streptococci, and enterococci including strains resistant to other marketed quinolones 1 These pathogens are frequently associated with respiratory diseases and skin infections Organisms show a low mutation rate to Q 1 OBJECTIVES MODELING & SIMULATION PLAN • Likely Q efficacious doses for IV dosing were predicted by simulation based on 4 data sets Specific objectives: • Q plasma protein binding characteristics in mouse and protein binding in man • The distribution of the PD target, Q AUC/MIC, (the pharmacodynamic index (PDI)) which was identified as the best predictor of efficacy in the mouse model of infection • 24 -hour steady-state Q AUC distribution for the human population of interest • Clinical isolate MIC distributions Develop a population PK model for Q administered IV Provide dose recommendation for future Phase II/III studies based on Q exposureresponse analyses for Exposure-efficacy response Exposure-tolerability responses Exposure-QTc prolongation response Exposure-LFT elevation response Exposure-serum creatinine elevation response To support the above analyses, develop respective exposure-response models and utilize these to simulate predicted responses for Q dosing regimens of interest Models developed from IV & oral phase I study data, respectively Final models were used to simulate subject level Q exposure measures for dosing regimens of interest The final Q PK model was a three compartment model consisting of a single central compartment and two peripheral compartments with zero-order absorption and first order elimination to and from the central compartment, respectively. Clearance was affected by sex and age. Peripheral volume V 2 was affected by weight and age. Peripheral volume V 3 was affected by weight, age and gender. Pharmacokinetic properties of Q facilitate human dosing 1 • Three pharmacodynamic index effects were simulated: stasis, 1 and 2 Log CFU killing • Q exposures in patients are expected to be higher than in healthy volunteers. However, patient Q PK was assumed to be equivalent to that of healthy volunteers. As a result, patient Q AUC estimates were conservative. • The probability of target effect (PTA) was calculated for each PDI effect, dose and fixed MICs. • The cumulative fraction of response (CFR) for each effect, dose and MIC distribution of interest was based on the PTA calculated for each MIC and the proportion of that MIC in that distribution • Minimum doses achieving a CFR ≥ 80% were considered representative of microbiological and clinical cure and thus likely efficacious doses EXPOSURE-SERUM CREATININE MODEL Overview of Efficacy M&S Methodology Pooled phase I IV dosing data was used to calculate and plot the median % change from baseline of serum creatinine as a function of dose and an Emax function of dose fitted to this data An established model of creatinine dynamics was implemented and linked to Q plasma levels under the assumption of Q mediated competitive inhibition of creatinine renal tubular secretion Resulting PK/PD model was then used to simulate the time course of serum creatinine elevation as a function of Q dosing regimen Effect is saturable and reversible upon cessation of dosing For gram-positive infections, 400 mg QD IV is predicted to be the lowest efficacious dose Typical Plots for Assessing Simulated Dose-Efficacy Relationships To date, 6 phase I clinical studies of Q have been completed in healthy volunteers 1 PK models were developed for IV and oral dosing: PK data from 5 distinct phase I studies in which Q was administered via IV infusion, QD 2 of these studies were single dose studies spanning the following doses: 10, 25, 50, 100, 200, 400, 600 & 800 mg The remaining studies were multiple dose studies of 14, 4 and 15 days duration spanning the following respective doses: 400 and 800 mg; and 800, 1200 and 1600 mg PK data from 1 phase I study in which Q was administered orally Multiple oral dosing for 12 days at doses of 400, 800 or 1200 mg Exposure-efficacy response analysis was based on Protein-binding in man and mouse and PDI values derived from a mouse thigh infection model Pooled demographic data from 5 phase I clinical studies (IV dosing) Clinical isolate MICs from a Q susceptibility survey Exposure-QTc prolongation response analysis was based on ECG data derived from 5 phase I studies (IV dosing) Exposure-serum creatinine elevation response analysis was based on Serum creatinine data from 4 of the 5 phase I IV dosing studies Exposure-LFT elevation response analysis was based on Data from the phase I oral dosing study • Q is predicted to have a narrow therapeutic window with an estimated minimum efficacious dose of 400 mg IV QD against Gram-positive organisms • Increasing Q dose provides relatively small efficacy benefits BUT at the expense of relatively large increases in QTc prolongation and the likelihood of LFT elevation • Serum creatinine elevations are saturated at estimated therapeutic doses and above CONCLUSIONS Based on these observation, Q may be an effective antibacterial agent in the treatment of gram-positive infections 1 PHASE I DATA OVERVIEW OF SIMULATED CLINICAL PROFILE Efficacy methodology was based on an established approach outlined in Ref 2 -5. Establish a quantitative framework to support Phase IIa dose selection for Q PHARMACOKINETIC MODEL EFFICACY MODEL Based on exposure-response analysis, the 400 mg dose is predicted to have an acceptable tolerability profile • Predicted maximum mean QTc prolongation at 400 mg is 9. 8 msec with a 90% confidence interval of 7. 1 to 13 msec • Modest risk of mild to moderate ALT/AST elevations • Serum creatinine elevations predicted to be saturable and near maximal and not to change with dose increases above 400 mg However, Q therapeutic window is likely to be narrow such that increasing Q dose from 400 mg to 800 mg is predicted to: EXPOSURE-LFT ELEVATION MODEL AST/ALT levels from oral dosing study graded by the NCI Common Terminology Criteria and day 13 levels encoded as 0 (no elevation) or 1 (grade 1 or greater) Logistic regression then used to model likelihood of day 13 elevation as a function of Q exposure measures Final model used to simulate AST/ALT elevation likelihood for dosing regimens EXPOSURE-QTc MODEL Focused on modeling the relationship between individually predicted Q plasma concentrations and, QTc. F, Fridericia corrected QT interval measurements. • Provide minimal improvements in efficacy against Gram-positive causative organisms • Almost double mean QTc prolongation resulting in borderline to unacceptable QTc outcomes • Approximately double the likelihood of ALT/AST elevation REFERENCES • Phase I oral dosing data 1. Daiichi Medical Research, 2006. Investigator’s Brochure, Version 3. 0. March 24 th, 2006 • Mild ALT elevations observed in the 400 and 800 mg treatment groups 2. Drusano GL, Preston, SL, Hardalo C, Hare, R, Banfield C, Andes D, Vesga O and Craig WA (2001). Use of preclinical data for selection of a Phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob. Agents Chemother. 45: 13 -22. • Mild and moderate ALT elevations observed in the 1200 mg group • Logistic regression used to model the likelihood of ALT elevation as a function of Q exposure measures 3. Drusano GL, Preston SL, Fowler C, Corrado M, Weisinger B & Kahn, J. (2004). Relationship between Fluoroquinolone Area under the Curve: Minimum Inhibitory Concentration Ratio and the Probability of Eradication of the Infecting Pathogen, in Patients with Nosocomial Pneumonia. The Journal of Infectious Diseases, 189: 1590– 7 • ALT elevations found to be well described by logistic regression functions of dose, steadystate Cmax, AUC or Cavg 4. Mouton JW, Dudley MN, Cars O, Derendorf H, and Drusano GL (2005). Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J. Antimicrob. Chemother. 55: 601 -607. Note: Solid lines denote lines of best fit, the shaded area the 90% confidence interval, symbols the observed mean incidence of ALT elevation and the error bars the standard error of the mean. All model parameter estimates and associated standard errors with corresponding t-values are listed in the table inserts. 5. Schentag JJ, Meagher AK, and Forrest A (2003). Fluoroquinolone AUIC break points and the link to bacterial killing rates. Part 2: human trials. Ann. Pharmacother. 37: 1478 -1488. 6. Kirschner D, Buchman T (2000). Mathematical modeling in surgical research. In: Surgical Research, Editors: Souba WW and Wilmore, D. Academic Press, San Diego, pp 1105 -1115. 7. Urakami Y, Kimura N, Okuda M, and Inui K-I (2004). Creatinine Transport by Basolateral Organic Transporter h. OCT 2 in the Human Kidney. Pharmaceutical Research, Volume 21, Number 6, June 2004, pp 976 -981(6).