Polypharmacy and Avoidng The Use of High Risk





















































- Slides: 94

Polypharmacy and Avoidng The Use of High Risk Medications June 13, 2014 Kristin Kim, RPh Omnicare Consultant Pharmacist Omnicare

Learning Objectives At the completion of this program, the participant will be able to: 1. State an appropriate definition of polypharmacy; 2. Define the term “unnecessary medications” and describe the risks associated with their use; 3. List specific modifiable and non-modifiable risk factors for polypharmacy; 4. Outline approaches and strategies for making drug therapy more appropriate; and 5. Determine necessary modifications to complex medication regimens involving common conditions seen in the long-term care setting.

Polypharmacy Facts Ø A study of 3000 nursing home residents found a rate of 1. 89 adverse drug events per 100 resident months. Overall, 51% of the events were judged to be preventable, with fatal, life-threatening, or serious events more likely to be preventable than less severe events. Most errors occur in the prescribing and monitoring phases of medication use. Ø Other studies indicate that from 30 – 64% of nursing home residents experience an adverse drug event. Ø Risk factors for developing adverse drug events have been identified and some are modifiable Gurwitz JH, Field TS, Avorn J, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med 2000; 109(2): 870 -94. Patient Safety at the Clinical Interface: Preliminary Meeting Summary. Organized by the Agency for Healthcare Research and Quality for the Quality Interagency Coordination Task Force. November 30, 2000. Field TS, Gurwitz JH, Avorn J et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med 2001; 161(13): 1629 -34.

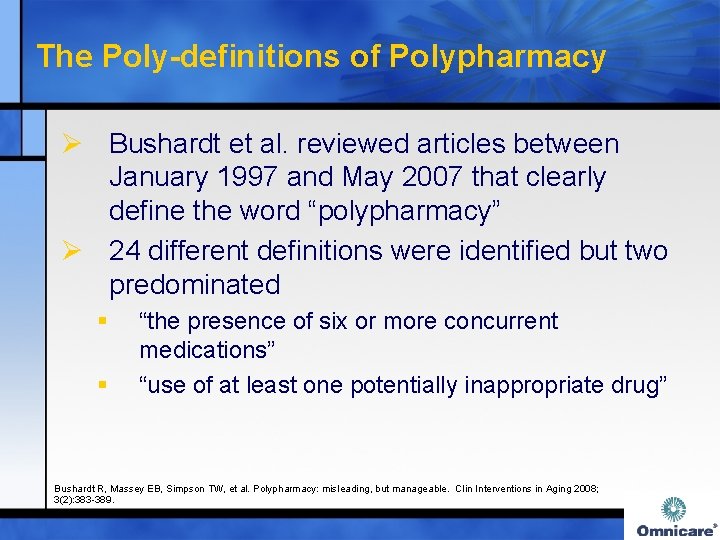
The Poly-definitions of Polypharmacy Ø Bushardt et al. reviewed articles between January 1997 and May 2007 that clearly define the word “polypharmacy” Ø 24 different definitions were identified but two predominated § § “the presence of six or more concurrent medications” “use of at least one potentially inappropriate drug” Bushardt R, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interventions in Aging 2008; 3(2): 383 -389.

So How to Define Polypharmacy? Ø A continued and growing health concern where more medications are prescribed than are warranted clinically Ø Polypharmacy should not be a quota on the number of medications prescribed concurrently For some residents, 1 medication may be too much and for others 15 medications may not be enough. Ø The goal is to provide the right number of medications and identify opportunities to eliminate unnecessary or potentially harmful drugs while adding drugs needed to assure optimal therapeutic outcomes.

Risks of Unnecessary Drug Use Ø F 329 specifically identifies the following risks: § § § Medication interactions Depression Confusion Immobility Falls and related hip fractures CFR 483. 25(I), Unnecessary Drugs

F 329 - What is an Unnecessary Drug? Ø According to F 329 “Each resident’s drug regimen must be free from unnecessary drugs. An unnecessary drug is any drug when used: (i) In excessive dose (including duplicate therapy); or (ii) For excessive duration; or (iii) Without adequate monitoring; or (iv) Without adequate indication for its use; or (v) In the presence of adverse consequences which indicate the dose should be reduced or discontinued; or (vi) Any combinations of the reasons above. ” CFR 483. 25(I), Unnecessary Drugs

Intent of F 329 Ø To help promote or maintain the resident’s highest practicable mental, physical, and psychosocial wellbeing: § § Use only those medications, in doses and for the duration clinically indicated to treat the resident’s assessed condition(s); Non-pharmacological interventions are considered and used when indicated, instead of, or in addition to, medication Clinically significant adverse consequences are minimized; and The potential contribution of the medication regimen to an unanticipated decline or newly emerging or worsening symptom is recognized and evaluated, and the regimen is modified when appropriate CFR 483. 25(I), Unnecessary Drugs

CMS Definitions Ø Excessive dose Ø Duplicate therapy Ø Excessive duration

Definitions – “Excessive dose” Ø “the total amount of any medication (including duplicate therapy) given at one time or over a period of time that is greater than the amount recommended by the manufacturer’s label, package insert, current standards of practice for resident’s age and condition, or clinical studies or evidence-based review articles that are published in medical and/or pharmacy journals and that lacks evidence of: § § § Ø A review for the continued necessity of the dose; Attempts at, or consideration of the possibility of, tapering a medication; and A documented clinical rationale for the benefit of, or necessity for, the dose or for the use of multiple medications from the same pharmacological class. ” “Factors influencing the appropriateness of any dose include the resident’s clinical response, possible adverse consequences, and other resident and medication-related variables” CFR 483. 25(I), Unnecessary Drugs

Definitions – “Duplicate therapy” Ø “Multiple medications of the same pharmacological class/category or any medication that substantially duplicates a particular effect of another medication that the individual is taking. ” Ø “Duplicate therapy is generally not indicated, unless current clinical standards of practice and documented clinical rationale confirm the benefits of multiple medications from the same class or with similar therapeutic effects. ” CFR 483. 25(I), Unnecessary Drugs

Definitions – “Excessive duration” Ø “Many conditions require treatment for extended periods, while others may resolve and no longer require medication therapy. ” Ø “The medication is administered beyond the manufacturer’s recommended time frames or facilityestablished stop order policies, beyond the length of time advised by current standards of practice, clinical practice guidelines, clinical studies or evidence-based review articles, and/or without either evidence of additional therapeutic benefit for the resident or clinical evidence that would warrant continued use of the medication” CFR 483. 25(I), Unnecessary Drugs

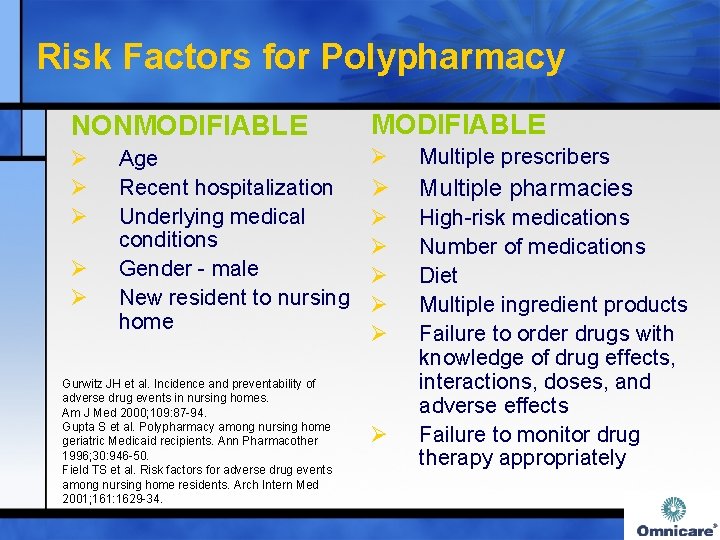
Risk Factors for Polypharmacy NONMODIFIABLE Ø Ø Multiple prescribers Ø Multiple pharmacies Ø Ø Ø High-risk medications Number of medications Diet Multiple ingredient products Failure to order drugs with knowledge of drug effects, interactions, doses, and adverse effects Failure to monitor drug therapy appropriately Ø Ø Age Recent hospitalization Underlying medical conditions Gender - male New resident to nursing home Gurwitz JH et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med 2000; 109: 87 -94. Gupta S et al. Polypharmacy among nursing home geriatric Medicaid recipients. Ann Pharmacother 1996; 30: 946 -50. Field TS et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med 2001; 161: 1629 -34. Ø

Costly Consequences of Polypharmacy In U. S. nursing homes in 1995 § § $3 billion dollars were spent on medications At least $4 billion dollars were spent on drug related problems § For every $1 dollar spent on drugs, $1. 33 is spent managing the complications of drug therapy!!! Without consultant pharmacist services, drug-related problems would be estimated at $7. 6 billion dollars § Bootman JL, Harrison DL, Cox E. The health care cost of drug-related morbidity and mortality in nursing facilities. Arch Intern Med 1997; 157: 2089 -96.

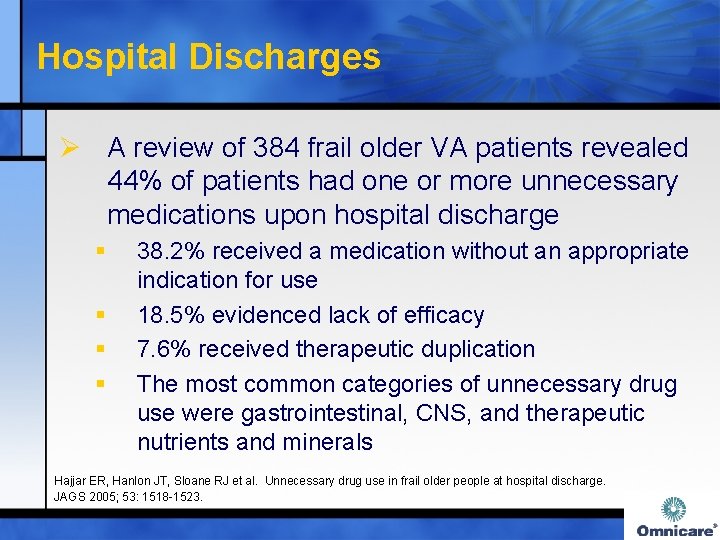
Hospital Discharges Ø A review of 384 frail older VA patients revealed 44% of patients had one or more unnecessary medications upon hospital discharge § § 38. 2% received a medication without an appropriate indication for use 18. 5% evidenced lack of efficacy 7. 6% received therapeutic duplication The most common categories of unnecessary drug use were gastrointestinal, CNS, and therapeutic nutrients and minerals Hajjar ER, Hanlon JT, Sloane RJ et al. Unnecessary drug use in frail older people at hospital discharge. JAGS 2005; 53: 1518 -1523.

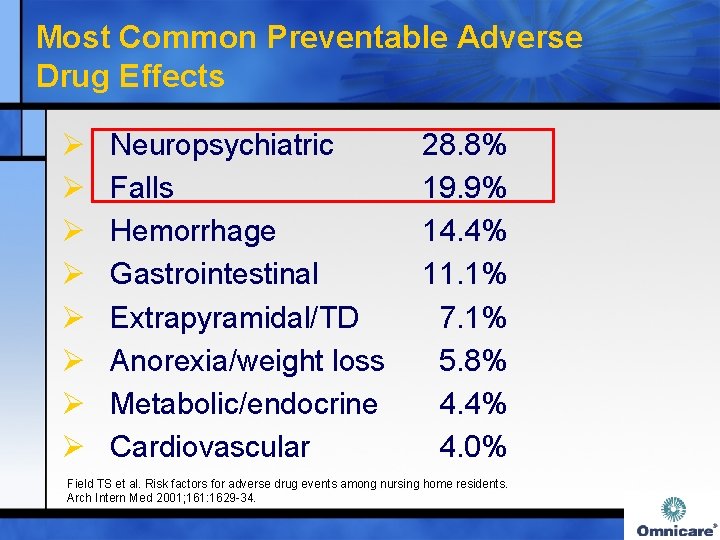
Most Common Preventable Adverse Drug Effects Ø Ø Ø Ø Neuropsychiatric Falls Hemorrhage Gastrointestinal Extrapyramidal/TD Anorexia/weight loss Metabolic/endocrine Cardiovascular 28. 8% 19. 9% 14. 4% 11. 1% 7. 1% 5. 8% 4. 4% 4. 0% Field TS et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med 2001; 161: 1629 -34.

Most Commonly Implicated Drug Classes in Preventable Events Odds Ratio* Opioids 6. 6 (2. 3 -19. 3) Antipsychotics 4. 0 (2. 2 -7. 3) Antibiotics/anti-infectives 3. 0 (1. 6 -5. 8) Anticonvulsants 2. 2 (1. 1 -4. 5) Antidepressants 2. 0 (1. 1 -3. 5) Nutrients/supplements 0. 27(0. 14 -0. 50) *(95% Confidence Interval) Field TS et al. Risk factors for adverse drug events among nursing home residents. Arch Intern Med 2001; 161: 1629 -34.

Fifteen Most Commonly Prescribed Potentially Inappropriate Drugs 1) 2) 3) 4) 5) 6) 7) Alprazolam Lorazepam Amitriptyline Nifedipine Promethazine Oxybutynin Fluoxetine 8) Cyclobenzaprine 9) Temazepam 10) Indomethacin 11) Amiodarone 12) Hydroxyzine 13) Diphenhydramine 14) Metaxalone 15) Naproxen Bushardt R, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interventions in Aging 2008; 3(2): 383 -389.

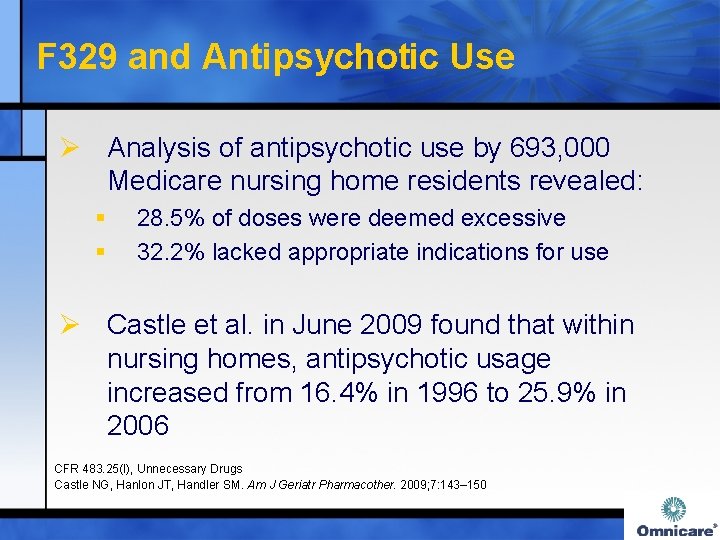
F 329 and Antipsychotic Use Ø Analysis of antipsychotic use by 693, 000 Medicare nursing home residents revealed: § § 28. 5% of doses were deemed excessive 32. 2% lacked appropriate indications for use Ø Castle et al. in June 2009 found that within nursing homes, antipsychotic usage increased from 16. 4% in 1996 to 25. 9% in 2006 CFR 483. 25(I), Unnecessary Drugs Castle NG, Hanlon JT, Handler SM. Am J Geriatr Pharmacother. 2009; 7: 143– 150

Additional Concerns with Antipsychotics Ø “Boxed Warning” on all antipsychotics, citing increased mortality when used in elderly patients with dementia-related psychosis Ø Numerous serious adverse effects, including stroke, diabetes, extrapyramidal symptoms, neuroleptic malignant syndrome, etc. Ø Even short-term use (e. g. , 30 days) of antipsychotic therapy has been associated with serious adverse events, including death Ray WA, Chung CP, Murray KT, et al. NEJM 2009; 360: 225 -35. Schneider LS, Tariot PN, Dagerman KS, et al. CATIE-AD. NEJM 2006; 355: 1525 -38. Castle NG, Hanlon JT, Handler SM. Am J Geriatr Pharmacother. 2009; 7: 143– 50

Discontinuation of Antipsychotics Ø Within the dementia antipsychotic withdrawal (DART-AD) trial, 64 patients (out of 128 total patients receiving antipsychotics) were randomized to receive placebo for 12 months Time point Probability of Survival: AP v. placebo 12 months 70% v. 77% 24 months 46% v. 71% 36 months 30% v. 59% Only 7 patients (10. 9%) required re-initiation of an antipsychotic after the 12 month trial was completed Ballard C, Hanney ML, Theodoulou M, et al. DART-AD: long-term follow-up of a randomised placebo-controlled trial. Lancet. 2009; 8(2): 151 -7.

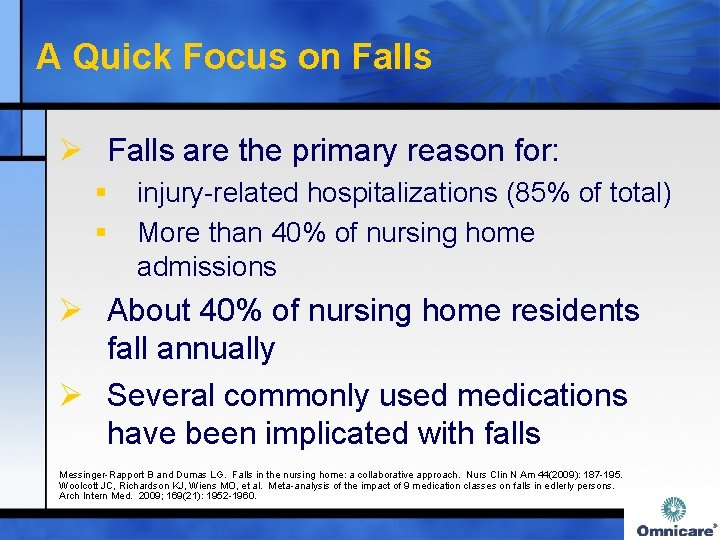
A Quick Focus on Falls Ø Falls are the primary reason for: § § injury-related hospitalizations (85% of total) More than 40% of nursing home admissions Ø About 40% of nursing home residents fall annually Ø Several commonly used medications have been implicated with falls Messinger-Rapport B and Dumas LG. Falls in the nursing home: a collaborative approach. Nurs Clin N Am 44(2009): 187 -195. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in edlerly persons. Arch Intern Med. 2009; 169(21): 1952 -1960.

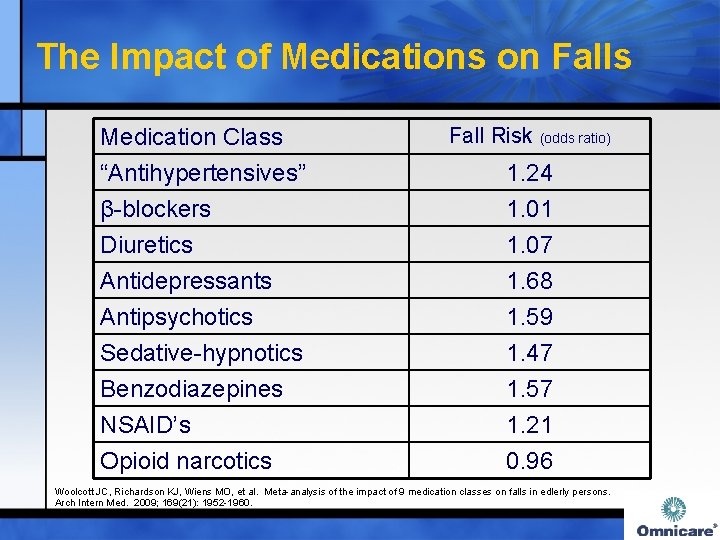
The Impact of Medications on Falls Medication Class “Antihypertensives” β-blockers Diuretics Fall Risk (odds ratio) Antidepressants Antipsychotics Sedative-hypnotics Benzodiazepines NSAID’s Opioid narcotics 1. 68 1. 59 1. 47 1. 57 1. 21 0. 96 1. 24 1. 01 1. 07 Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in edlerly persons. Arch Intern Med. 2009; 169(21): 1952 -1960.

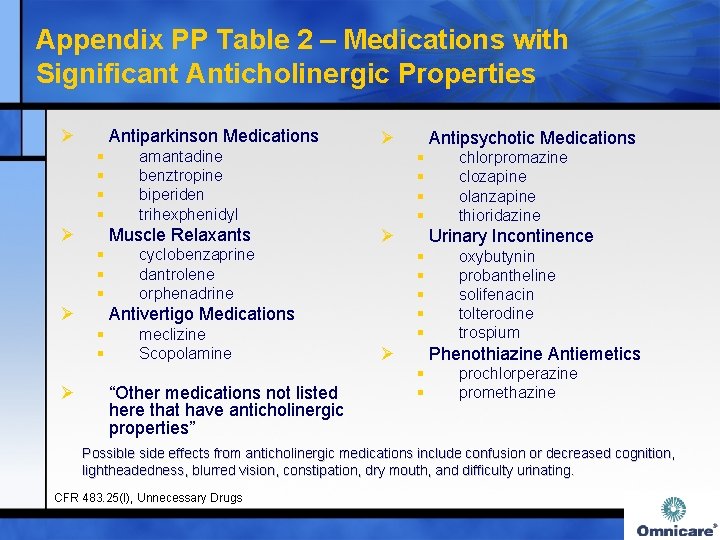
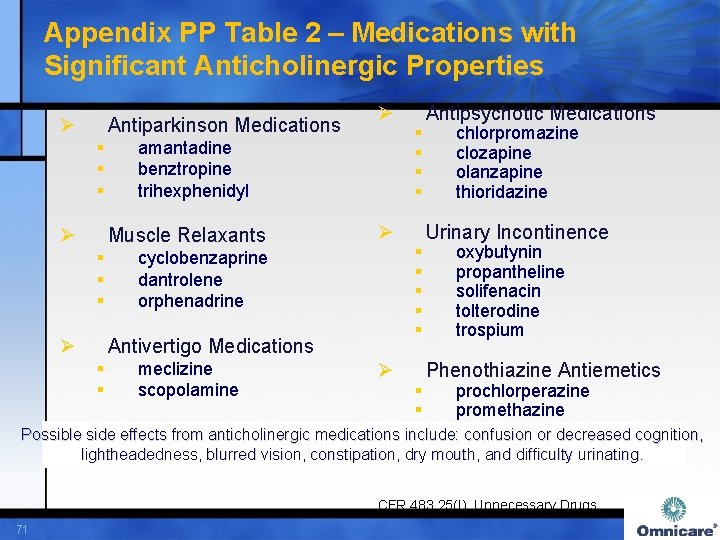
Appendix PP Table 2 – Medications with Significant Anticholinergic Properties Ø Antiparkinson Medications § § Ø Muscle Relaxants § § § Ø cyclobenzaprine dantrolene orphenadrine meclizine Scopolamine “Other medications not listed here that have anticholinergic properties” Antipsychotic Medications § § Ø chlorpromazine clozapine olanzapine thioridazine Urinary Incontinence § § § Antivertigo Medications § § Ø amantadine benztropine biperiden trihexphenidyl Ø Ø oxybutynin probantheline solifenacin tolterodine trospium Phenothiazine Antiemetics § § prochlorperazine promethazine Possible side effects from anticholinergic medications include confusion or decreased cognition, lightheadedness, blurred vision, constipation, dry mouth, and difficulty urinating. CFR 483. 25(I), Unnecessary Drugs

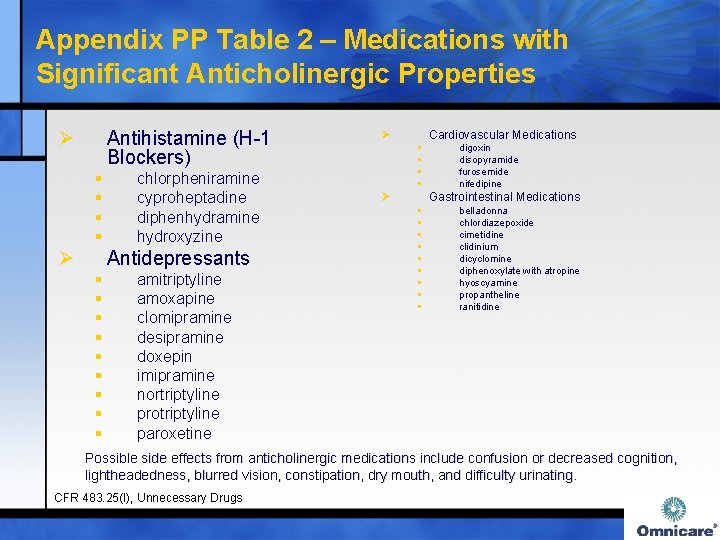
Appendix PP Table 2 – Medications with Significant Anticholinergic Properties Ø Antihistamine (H-1 Blockers) § § Ø chlorpheniramine cyproheptadine diphenhydramine hydroxyzine Antidepressants § § § § § amitriptyline amoxapine clomipramine desipramine doxepin imipramine nortriptyline protriptyline paroxetine Ø Cardiovascular Medications § § Ø digoxin disopyramide furosemide nifedipine Gastrointestinal Medications § § § § § belladonna chlordiazepoxide cimetidine clidinium dicyclomine diphenoxylate with atropine hyoscyamine propantheline ranitidine Possible side effects from anticholinergic medications include confusion or decreased cognition, lightheadedness, blurred vision, constipation, dry mouth, and difficulty urinating. CFR 483. 25(I), Unnecessary Drugs

Approaches to Helping Make Drug Therapy Appropriate Ø ARMOR Ø STOPP Ø START

ARMOR (Assess Review Minimize Optimize Reassess) Ø Assess § § Ø total number of medications Use of medications with higher rates of adverse outcomes (e. g. , β-blockers, psychotropics, pain medications, Beers Criteria medications) Review § § Ø drug-drug interactions drug-disease interactions impact on functional status benefits versus risk of therapy (e. g. , weight changes, bladder changes, skin changes, etc) Minimize (non-essential medications) § § lack of clear indication risk outweighs benefits Haque R. ARMOR: A tool to evaluate polypharmacy in elderly persons. Ann LTC 2009; 17(6): 26 -30.

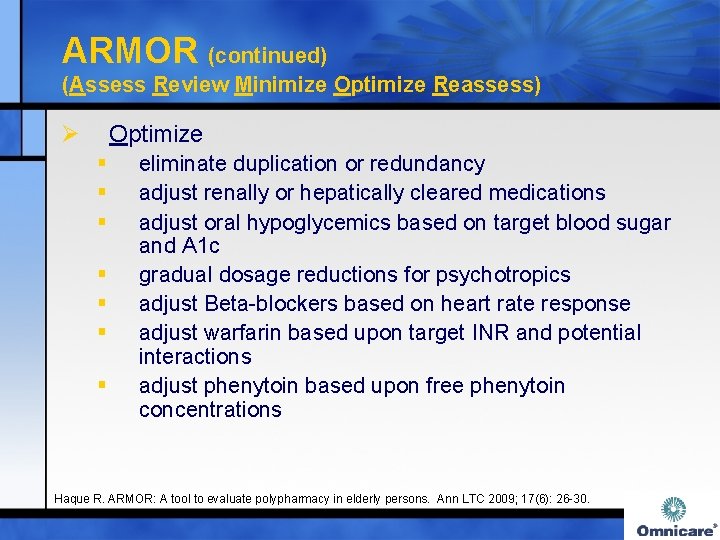
ARMOR (continued) (Assess Review Minimize Optimize Reassess) Ø Optimize § § § § eliminate duplication or redundancy adjust renally or hepatically cleared medications adjust oral hypoglycemics based on target blood sugar and A 1 c gradual dosage reductions for psychotropics adjust Beta-blockers based on heart rate response adjust warfarin based upon target INR and potential interactions adjust phenytoin based upon free phenytoin concentrations Haque R. ARMOR: A tool to evaluate polypharmacy in elderly persons. Ann LTC 2009; 17(6): 26 -30.

ARMOR (continued) (Assess Review Minimize Optimize Reassess) Ø Reassess § § § vitals at rest and during activity functional status cognitive status clinical status medication compliance Haque R. ARMOR: A tool to evaluate polypharmacy in elderly persons. Ann LTC 2009; 17(6): 26 -30.

STOPP – Screening Tool of Older People’s potentially inappropriate Prescriptions Ø 65 categories of potentially inappropriate prescribing in persons aged 65 years or older (33 categories more than Beers’ Criteria) Ø Compared to Beers’ Criteria, STOPP identified: § § 336 potentially inappropriate medicines (PIMs) affecting 247 patients (35%) compared to 226 PIMs affecting 177 patients (25%) a significantly higher proportion of patients requiring hospitalization due to a PIM-related adverse event (91% v. 48%) Gallagher P, Ryan C, Byrne S, et al. STOPP and START – consensus validation. Int J Clin Pharmacol Ther. 2008 46(2): 72 -83. Gallagher P and O’Mahony D. STOPP: Application to acutely ill elderly patients and comparison with Beers’ criteria. Age and Ageing 2008: 1 -7.

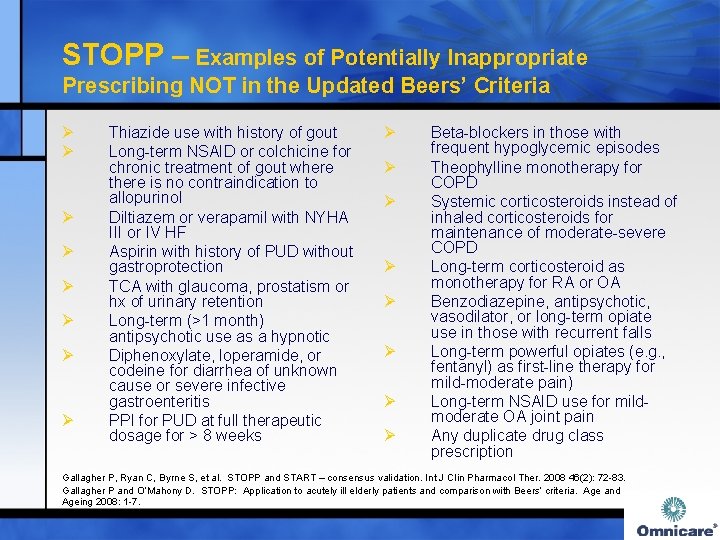
STOPP – Examples of Potentially Inappropriate Prescribing NOT in the Updated Beers’ Criteria Ø Ø Ø Ø Thiazide use with history of gout Long-term NSAID or colchicine for chronic treatment of gout where there is no contraindication to allopurinol Diltiazem or verapamil with NYHA III or IV HF Aspirin with history of PUD without gastroprotection TCA with glaucoma, prostatism or hx of urinary retention Long-term (>1 month) antipsychotic use as a hypnotic Diphenoxylate, loperamide, or codeine for diarrhea of unknown cause or severe infective gastroenteritis PPI for PUD at full therapeutic dosage for > 8 weeks Ø Ø Ø Ø Beta-blockers in those with frequent hypoglycemic episodes Theophylline monotherapy for COPD Systemic corticosteroids instead of inhaled corticosteroids for maintenance of moderate-severe COPD Long-term corticosteroid as monotherapy for RA or OA Benzodiazepine, antipsychotic, vasodilator, or long-term opiate use in those with recurrent falls Long-term powerful opiates (e. g. , fentanyl) as first-line therapy for mild-moderate pain) Long-term NSAID use for mildmoderate OA joint pain Any duplicate drug class prescription Gallagher P, Ryan C, Byrne S, et al. STOPP and START – consensus validation. Int J Clin Pharmacol Ther. 2008 46(2): 72 -83. Gallagher P and O’Mahony D. STOPP: Application to acutely ill elderly patients and comparison with Beers’ criteria. Age and Ageing 2008: 1 -7.

STOPP – Screening Tool of Older People’s potentially inappropriate Prescriptions Ø Most commonly observed PIMs § § § Long acting benzodiazepines or tricyclic antidepressants with a contraindication Prolonged use of first generation antihistamines Medications that increase falls in patients already known to be prone to falling (e. g. , benzodiazepines, antipsychotics, vasodilators) Inappropriate use of NSAIDs and opioids Duplicate therapy (e. g. , 2 ACE Inhibitors, 2 NSAIDs, 2 SSRIs, 2 antiplatelet therapies) Gallagher P, Ryan C, Byrne S, et al. STOPP and START – consensus validation. Int J Clin Pharmacol Ther. 2008 46(2): 72 -83. Gallagher P and O’Mahony D. STOPP: Application to acutely ill elderly patients and comparison with Beers’ criteria. Age and Ageing 2008: 1 -7.

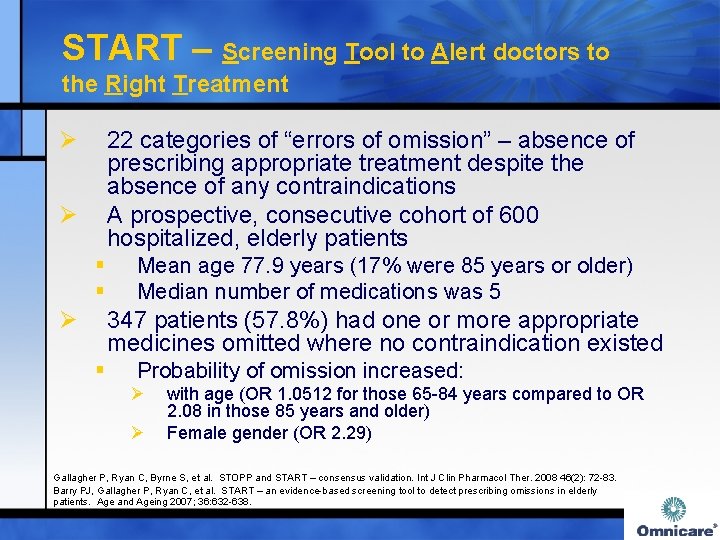
START – Screening Tool to Alert doctors to the Right Treatment Ø 22 categories of “errors of omission” – absence of prescribing appropriate treatment despite the absence of any contraindications A prospective, consecutive cohort of 600 hospitalized, elderly patients Ø § § Ø Mean age 77. 9 years (17% were 85 years or older) Median number of medications was 5 347 patients (57. 8%) had one or more appropriate medicines omitted where no contraindication existed § Probability of omission increased: Ø Ø with age (OR 1. 0512 for those 65 -84 years compared to OR 2. 08 in those 85 years and older) Female gender (OR 2. 29) Gallagher P, Ryan C, Byrne S, et al. STOPP and START – consensus validation. Int J Clin Pharmacol Ther. 2008 46(2): 72 -83. Barry PJ, Gallagher P, Ryan C, et al. START – an evidence-based screening tool to detect prescribing omissions in elderly patients. Age and Ageing 2007; 36: 632 -638.

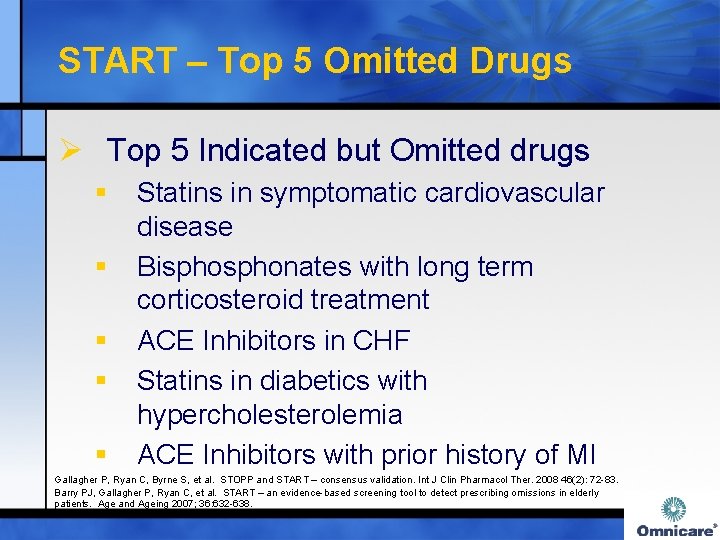
START – Top 5 Omitted Drugs Ø Top 5 Indicated but Omitted drugs § § § Statins in symptomatic cardiovascular disease Bisphonates with long term corticosteroid treatment ACE Inhibitors in CHF Statins in diabetics with hypercholesterolemia ACE Inhibitors with prior history of MI Gallagher P, Ryan C, Byrne S, et al. STOPP and START – consensus validation. Int J Clin Pharmacol Ther. 2008 46(2): 72 -83. Barry PJ, Gallagher P, Ryan C, et al. START – an evidence-based screening tool to detect prescribing omissions in elderly patients. Age and Ageing 2007; 36: 632 -638.

START – Other Categories of Omissions Ø Ø Ø Warfarin in the presence of chronic A-Fib (or aspirin when warfarin was contraindicated) Aspirin with a documented history of coronary, cerebral, or peripheral vascular disease OR diabetes with well controlled blood pressure Calcium and vitamin D for osteoporosis Routine inhaled beta-agonist or anticholinergic in mild to moderate asthma or COPD Routine inhaled corticosteroid in moderate-severe asthma or COPD ACE Inhibitors in diabetics with overt proteinuria or microalbuminuria Antidepressant therapy in the presence of “clear-cut” depressive symptoms Levodopa/carbidopa in Parkinson’s with definite functional impairment Antihypertensive therapy in clearly defined hypertension Fiber supplementation in chronic, symptomatic diverticular disease with constipation PPI in the presence of chronic severe GERD Gallagher P, Ryan C, Byrne S, et al. STOPP and START – consensus validation. Int J Clin Pharmacol Ther. 2008 46(2): 72 -83. Barry PJ, Gallagher P, Ryan C, et al. START – an evidence-based screening tool to detect prescribing omissions in elderly patients. Age and Ageing 2007; 36: 632 -638.


Principles of Medication Safety Medication safety spans all phases of the medication use process including prescribing, transcribing, dispensing, administration, and monitoring of medications. Failure at any step along the medication use continuum can result in a failed therapeutic response, toxicity, and unnecessary expense.

General Guidelines for Preventing Medication Therapy Problems Evaluate elderly patients thoroughly. • Manage medical conditions without drugs as often as possible. • Know the pharmacology of the drug being prescribed and how it might adversely interact with other drugs. • Consider how the clinical status of each patient could influence the pharmacology and effectiveness of the drug(s). • Be sensitive to potential barriers to compliance (e. g. , impaired cognitive function, diminished vision and hearing, cultural barriers) • For drugs or their active metabolites that are renally eliminated, make appropriate age-related adjustments in dosages. • If there is a question about drug dosage, start small and increase gradually. • Use drug blood concentrations to monitor potentially toxic drugs used frequently in the elderly. • Monitor elderly patients frequently for compliance, drug effects and toxicity. Kane, R. L. , Ouslander, J. G. , & Abrass, I. B. (1994). Essentials of clinical geriatrics. New York: Mc. Graw-Hill. p. 373.

Medication Appropriateness Checklist Hanlon JT, Schmader KE et al. J Clin Epidemiol 1992 ; 45 : 1045 Fitzgerald LS, Hanlon JT et al. Ann Pharmacother 1997 ; 31 : 543 -8. Schmader KE. Hanlon JT. Pieper CF et al. Am J Med 2004; 116(6): 394 -401.

Questions That May Help Improve Drug Use Ø Ø Ø Ø Has the disease state resolved that the drug was originally prescribed for? Are non-pharmacological interventions being used or are there alternative non-drug options that could be tried? Is there another drug more effective for the disease? Is there an equally effective, lower-cost drug available? Have treatment goals been achieved? Are there any adverse effects to the medication observed? Are 2+ drugs of the same class/pharmacological action being used? Are there any drug-drug or drug-disease interactions? Is there a lower effective dose of the medication? Are any medications dosed more than 2 times per day? Are there any adherence / compliance concerns noted? Should the dosage be adjusted based on age, renal, or liver status? Has there been any recent change in IADL or ADL status? Bushardt R, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interventions in Aging 2008; 3(2): 383 -389.

Discontinuing Medications Ø In a study of 124 ambulatory elderly, undergoing 238 drug discontinuations, 26% experienced an adverse drug event related to drug discontinuation Ø Most common problems occurred with stopping cardiovascular or CNS drugs Ø 88% were attributed to exacerbation of underlying disease Ø 80% of drug discontinuations were NOT restarted Ø 36% resulted in hospitalization Graves T, Hanlon J, Schmader K. Adverse events after discontinuing medications in elderly outpatients. Arch Intern Med 1997; 157: 2205 -10.

Improving Drug Efficiency Ø Look for once daily and extended-release formulations to decrease the number of administration times and minimize peak to trough variation in serum concentrations Ø Consider use of combination drug products in replacement of the two individual components Ø Evaluate if the dose can be decreased to minimize the likelihood of adverse events without compromising efficacy Ø Assure that optimal monitoring strategies are in place to evaluate therapeutic success and potential toxicity

Application to Common Conditions Ø Ø Ø Ø Alzheimer’s Asthma/COPD Behavioral Problems Benign Prostatic Hyperplasia Chronic Kidney Disease Depression Diabetes Mellitus GERD Ø Ø Ø Ø Ø Heart Failure Hypertension Insomnia Lipid Disorders Osteoporosis Pain Syndromes Seizure Disorder Stroke Urinary Incontinence Weight Loss/Anorexia

Application to Common Conditions – Alzheimer’s Disease Scenario Why is this Potentially Inappropriate? Acetylcholinesterase inhibitors (donepezil, galantamine, or rivastigmine) + anticholinergic agents Pharmacological antagonism and deleterious effects on cognition Acetylcholinesterase inhibitors with benzodiazepines Worsening cognition related to additive CNS adverse effects Acetylcholinesterase inhibitors Increased risk of cardiac with cardiac conduction disorder conduction abnormalities CFR 483. 25(I), Unnecessary Drugs Arch Intern Med 2003; 163: 2716 -24 Am J Geriatr Psych 2003; 11: 458 -461

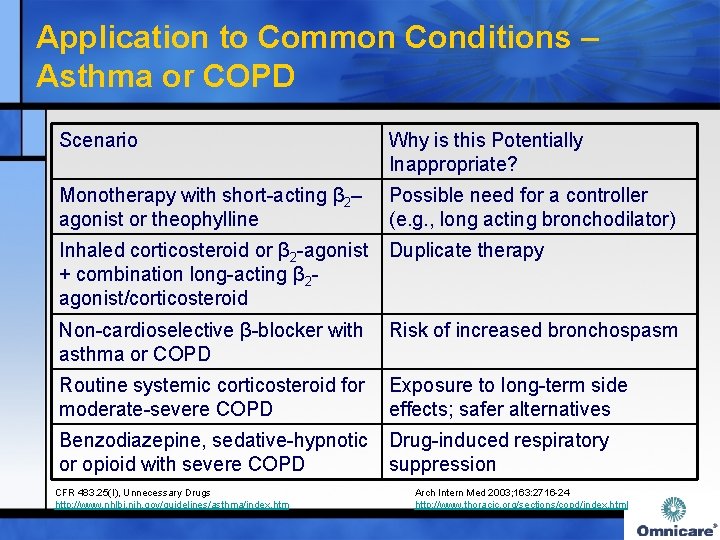
Application to Common Conditions – Asthma or COPD Scenario Why is this Potentially Inappropriate? Monotherapy with short-acting β 2– agonist or theophylline Possible need for a controller (e. g. , long acting bronchodilator) Inhaled corticosteroid or β 2 -agonist Duplicate therapy + combination long-acting β 2 agonist/corticosteroid Non-cardioselective β-blocker with Risk of increased bronchospasm asthma or COPD Routine systemic corticosteroid for Exposure to long-term side moderate-severe COPD effects; safer alternatives Benzodiazepine, sedative-hypnotic Drug-induced respiratory or opioid with severe COPD suppression CFR 483. 25(I), Unnecessary Drugs http: //www. nhlbi. nih. gov/guidelines/asthma/index. htm Arch Intern Med 2003; 163: 2716 -24 http: //www. thoracic. org/sections/copd/index. html

Application to Common Conditions – Behavioral Problems Scenario Why is this Potentially Inappropriate? Duplicate anxiolytics, hypnotics, antipsychotics, antidepressants Additive CNS effects Daily dose of Exposure to excessive dose psychopharmacological medication increases risk of adverse exceeding thresholds established in consequences F 329 - Table 1 Antipsychotic + anticholinergic (e. g. , “Cascade effect” –treating drugbenztropine) induced side effect Metoclopramide + antipsychotic CFR 483. 25(I), Unnecessary Drugs Arch Intern Med 2003; 163: 2716 -24 Increased risk of abnormal involuntary movements

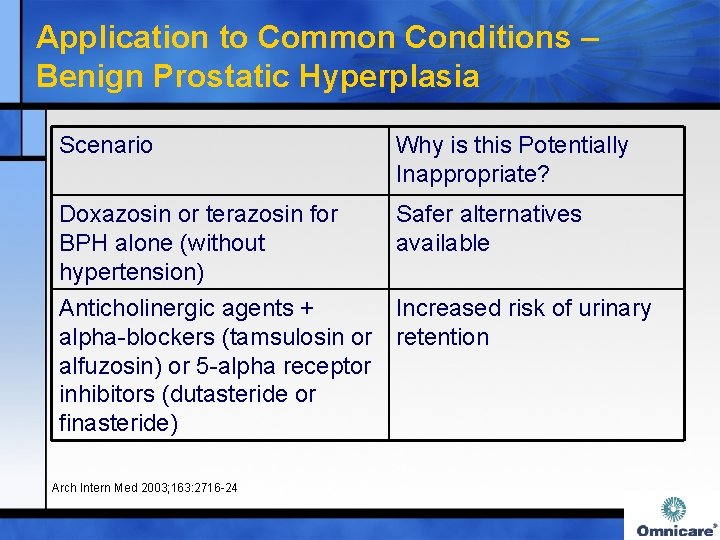
Application to Common Conditions – Benign Prostatic Hyperplasia Scenario Why is this Potentially Inappropriate? Doxazosin or terazosin for BPH alone (without hypertension) Safer alternatives available Anticholinergic agents + Increased risk of urinary alpha-blockers (tamsulosin or retention alfuzosin) or 5 -alpha receptor inhibitors (dutasteride or finasteride) Arch Intern Med 2003; 163: 2716 -24

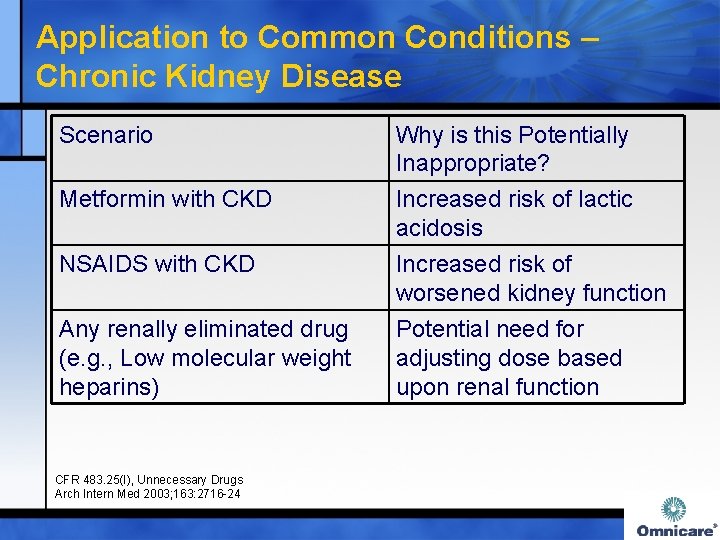
Application to Common Conditions – Chronic Kidney Disease Scenario Metformin with CKD Why is this Potentially Inappropriate? Increased risk of lactic acidosis NSAIDS with CKD Increased risk of worsened kidney function Any renally eliminated drug (e. g. , Low molecular weight heparins) Potential need for adjusting dose based upon renal function CFR 483. 25(I), Unnecessary Drugs Arch Intern Med 2003; 163: 2716 -24

Renal Adjustment of Selected Medications Ø Ø Ø Ø Ø Acyclovir Amantadine Chlorpropamide Ciprofloxacin Colchicine Gabapentin Glyburide Memantine Meperidine Nitrofurantoin Ø Ø Ø Ø Probenecid Propoxyphene Ranitidine Rimantidine Spironolactone Sulfamethoxazole / trimethoprim Triamterene Valacyclovir - This list is not all inclusive of medications that require renal dosing Hanlon JT, Aspinall SL, Semla TP, et al. Consensus Guidelines for Oral Dosing of Primarily Renally Cleared Medications in Older Adults. J Am Geriatr Soc. 2009 Feb; 57(2): 335 -40.

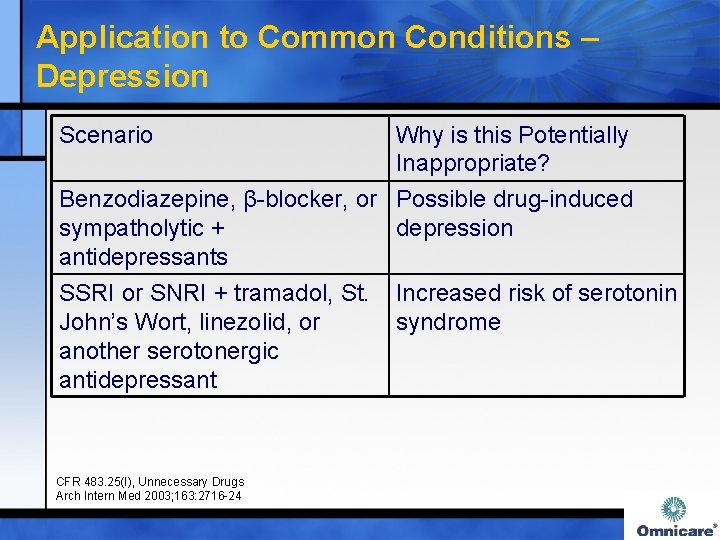
Application to Common Conditions – Depression Scenario Why is this Potentially Inappropriate? Benzodiazepine, β-blocker, or Possible drug-induced sympatholytic + depression antidepressants SSRI or SNRI + tramadol, St. Increased risk of serotonin John’s Wort, linezolid, or syndrome another serotonergic antidepressant CFR 483. 25(I), Unnecessary Drugs Arch Intern Med 2003; 163: 2716 -24

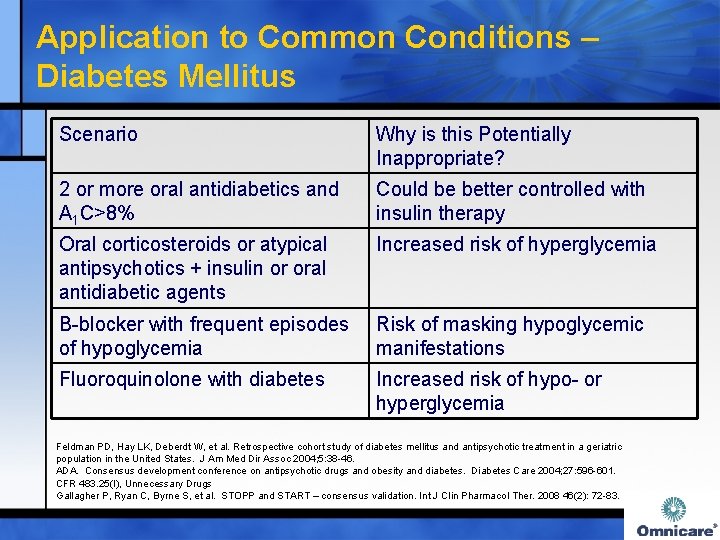
Application to Common Conditions – Diabetes Mellitus Scenario Why is this Potentially Inappropriate? 2 or more oral antidiabetics and A 1 C>8% Could be better controlled with insulin therapy Oral corticosteroids or atypical antipsychotics + insulin or oral antidiabetic agents Increased risk of hyperglycemia Β-blocker with frequent episodes of hypoglycemia Risk of masking hypoglycemic manifestations Fluoroquinolone with diabetes Increased risk of hypo- or hyperglycemia Feldman PD, Hay LK, Deberdt W, et al. Retrospective cohort study of diabetes mellitus and antipsychotic treatment in a geriatric population in the United States. J Am Med Dir Assoc 2004; 5: 38 -46. ADA. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 27: 596 -601. CFR 483. 25(I), Unnecessary Drugs Gallagher P, Ryan C, Byrne S, et al. STOPP and START – consensus validation. Int J Clin Pharmacol Ther. 2008 46(2): 72 -83.

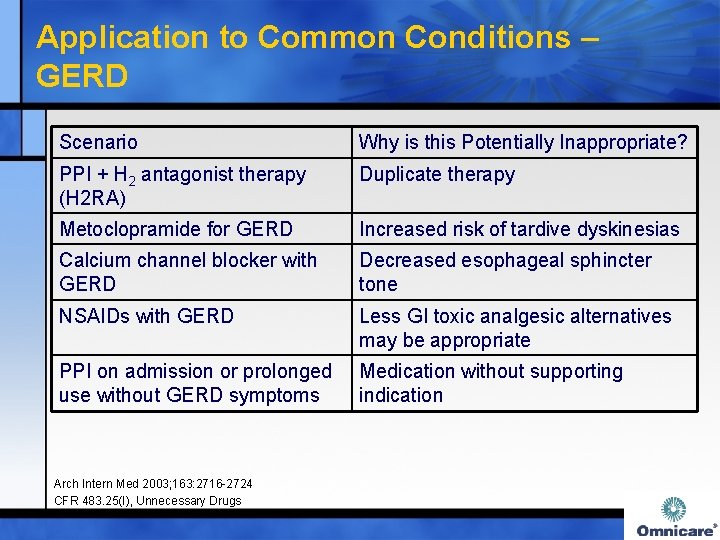
Application to Common Conditions – GERD Scenario Why is this Potentially Inappropriate? PPI + H 2 antagonist therapy (H 2 RA) Duplicate therapy Metoclopramide for GERD Increased risk of tardive dyskinesias Calcium channel blocker with GERD Decreased esophageal sphincter tone NSAIDs with GERD Less GI toxic analgesic alternatives may be appropriate PPI on admission or prolonged use without GERD symptoms Medication without supporting indication Arch Intern Med 2003; 163: 2716 -2724 CFR 483. 25(I), Unnecessary Drugs

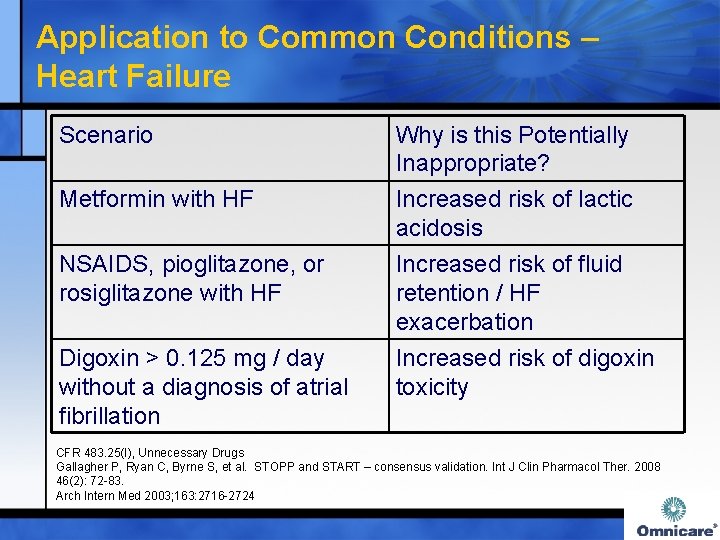
Application to Common Conditions – Heart Failure Scenario Metformin with HF NSAIDS, pioglitazone, or rosiglitazone with HF Digoxin > 0. 125 mg / day without a diagnosis of atrial fibrillation Why is this Potentially Inappropriate? Increased risk of lactic acidosis Increased risk of fluid retention / HF exacerbation Increased risk of digoxin toxicity CFR 483. 25(I), Unnecessary Drugs Gallagher P, Ryan C, Byrne S, et al. STOPP and START – consensus validation. Int J Clin Pharmacol Ther. 2008 46(2): 72 -83. Arch Intern Med 2003; 163: 2716 -2724

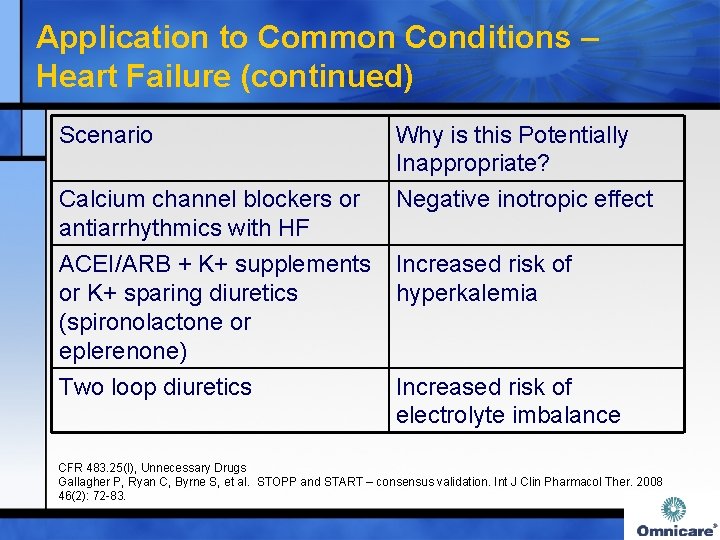
Application to Common Conditions – Heart Failure (continued) Scenario Why is this Potentially Inappropriate? Negative inotropic effect Calcium channel blockers or antiarrhythmics with HF ACEI/ARB + K+ supplements Increased risk of or K+ sparing diuretics hyperkalemia (spironolactone or eplerenone) Two loop diuretics Increased risk of electrolyte imbalance CFR 483. 25(I), Unnecessary Drugs Gallagher P, Ryan C, Byrne S, et al. STOPP and START – consensus validation. Int J Clin Pharmacol Ther. 2008 46(2): 72 -83.

Application to Common Conditions – Hypertension Scenario NSAIDs or pioglitazone or rosiglitazone + antihypertensives Why is this Potentially Inappropriate? Increased blood pressure due to fluid retention Verapamil + anticholinergics Additive anticholinergic effects Reserpine > 0. 25 mg/day Increased risk of depression, impotence, sedation, and orthostatic hypotension CFR 483. 25(I), Unnecessary Drugs Gallagher P, Ryan C, Byrne S, et al. STOPP and START – consensus validation. Int J Clin Pharmacol Ther. 2008 46(2): 72 -83. Arch Intern Med 2003; 163: 2716 -2724

Application to Common Conditions – Hypertension (continued) Scenario Why is this Potentially Inappropriate? Methyldopa, clonidine, doxazosin, terazosin Better options available for the elderly CNS stimulants, decongestants, or erythropoiesis stimulating agent with hypertension Increased blood pressure 4 or more concurrent antihypertensives increased cost and potential for adverse consequences Arch Intern Med 2003; 163: 2716 -2724 CFR 483. 25(I), Unnecessary Drugs

Application to Common Conditions – Insomnia Scenario Why is this Potentially Inappropriate? Decongestants, theophylline, methylphenidate, MAOIs, SSRIs with insomnia Daily dose or duration of sedative-hypnotic exceeds thresholds established in F 329 - Table 1 May cause or worsen insomnia CFR 483. 25(I), Unnecessary Drugs Arch Intern Med 2003; 163: 2716 -24 Excessive dose and/or duration

Application to Common Conditions – Lipid Disorders Scenario Why is this Potentially Inappropriate? Cholestyramine + other medications Reduced absorption of other meds (administer them 1 hour before or 4 hours after) Statin but not at LDL/triglyceride goal CFR 483. 25(I), Unnecessary Drugs NCEP/ATPIII JAMA 2001; 285: 2486 Potential need for dosage increase

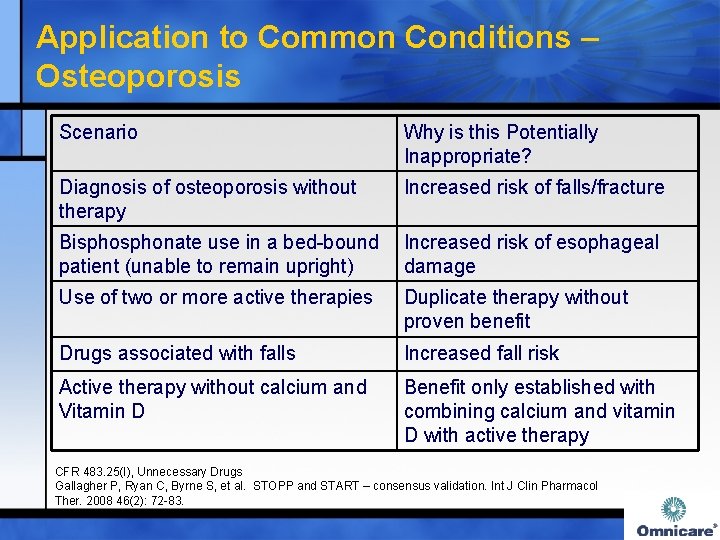
Application to Common Conditions – Osteoporosis Scenario Why is this Potentially Inappropriate? Diagnosis of osteoporosis without therapy Increased risk of falls/fracture Bisphonate use in a bed-bound Increased risk of esophageal patient (unable to remain upright) damage Use of two or more active therapies Duplicate therapy without proven benefit Drugs associated with falls Increased fall risk Active therapy without calcium and Vitamin D Benefit only established with combining calcium and vitamin D with active therapy CFR 483. 25(I), Unnecessary Drugs Gallagher P, Ryan C, Byrne S, et al. STOPP and START – consensus validation. Int J Clin Pharmacol Ther. 2008 46(2): 72 -83.

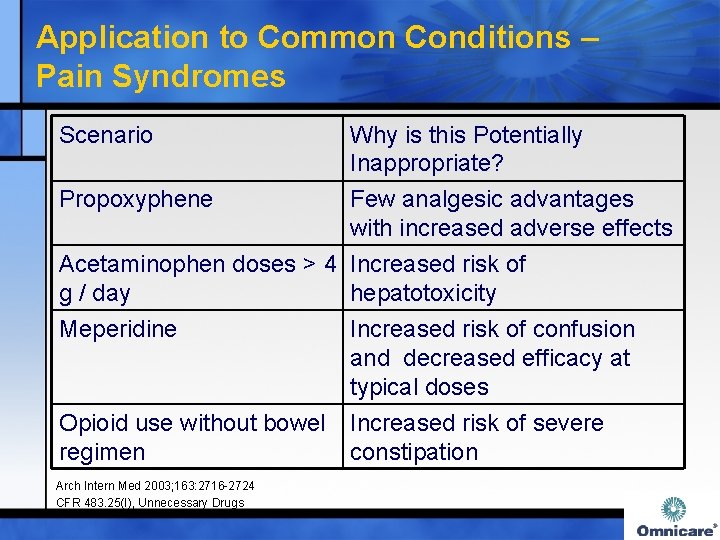
Application to Common Conditions – Pain Syndromes Scenario Why is this Potentially Inappropriate? Propoxyphene Few analgesic advantages with increased adverse effects Acetaminophen doses > 4 Increased risk of g / day hepatotoxicity Meperidine Increased risk of confusion and decreased efficacy at typical doses Opioid use without bowel Increased risk of severe regimen constipation Arch Intern Med 2003; 163: 2716 -2724 CFR 483. 25(I), Unnecessary Drugs

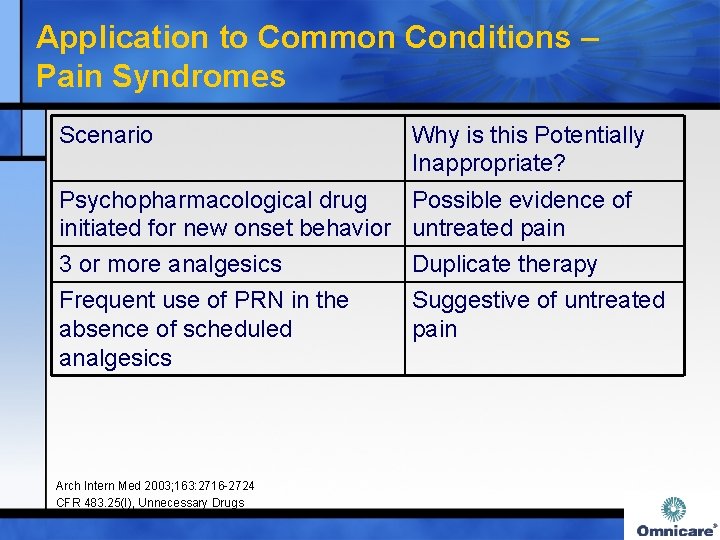
Application to Common Conditions – Pain Syndromes Scenario Why is this Potentially Inappropriate? Psychopharmacological drug Possible evidence of initiated for new onset behavior untreated pain 3 or more analgesics Duplicate therapy Frequent use of PRN in the absence of scheduled analgesics Suggestive of untreated pain Arch Intern Med 2003; 163: 2716 -2724 CFR 483. 25(I), Unnecessary Drugs

Application to Common Conditions – Seizure Disorder Scenario Why is this Potentially Inappropriate? Antipyschotics, bupropion, Lowered seizure threshold fluoroquinolones, meperidine, metoclopramide, promethazine, prochlorperazine, tramadol, or tricyclic antidepressant with seizure disorder or use of anticonvulsants Long-term anticonvulsant therapy at Re-evaluate seizure history / very subtherapeutic doses and indication (excessive duration serum concentrations of prophylaxis? ) CFR 483. 25(I), Unnecessary Drugs Arch Intern Med 2003; 163: 2716 -2724

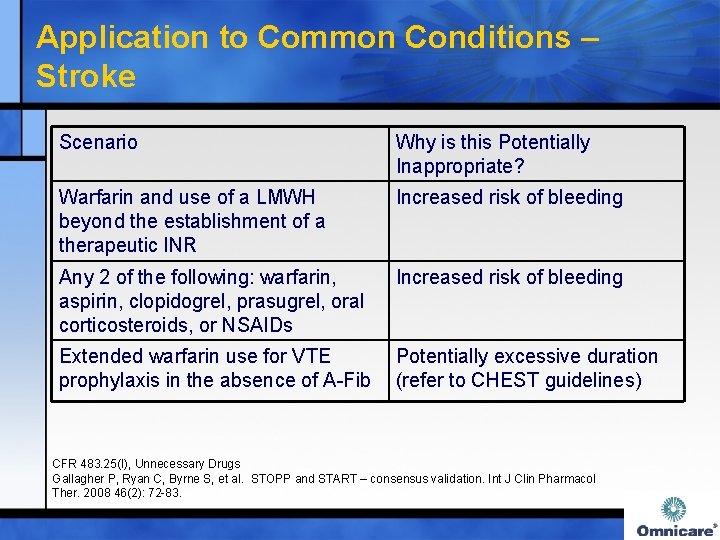
Application to Common Conditions – Stroke Scenario Why is this Potentially Inappropriate? Warfarin and use of a LMWH beyond the establishment of a therapeutic INR Increased risk of bleeding Any 2 of the following: warfarin, aspirin, clopidogrel, prasugrel, oral corticosteroids, or NSAIDs Increased risk of bleeding Extended warfarin use for VTE Potentially excessive duration prophylaxis in the absence of A-Fib (refer to CHEST guidelines) CFR 483. 25(I), Unnecessary Drugs Gallagher P, Ryan C, Byrne S, et al. STOPP and START – consensus validation. Int J Clin Pharmacol Ther. 2008 46(2): 72 -83.

Application to Common Conditions – Urinary Incontinence Scenario Why is this Potentially Inappropriate? Late-day/evening administration of diuretics Increased risk of nocturnal falls OAB medications (e. g. , oxybutynin ER, tolterodine, etc. ) + anticholinergic medications Decreased urinary flow and increased risk of urinary retention CFR 483. 25(I), Unnecessary Drugs Arch Intern Med 2003; 163: 2716 -24

Application to Common Conditions – Weight Loss / Anorexia Scenario Why is this Potentially Inappropriate? Acetylcholinesterase inhibitor, Potential drug-induced digoxin, fluoxetine or CNS weight loss stimulants + megesterol, cyproheptadine, oxandrolone or mirtazapine Arch Intern Med 2003; 163: 2716 -24

Summary Ø Polypharmacy is not a new concept Ø All drug therapy must be individualized to the individual patient Ø Consultant pharmacists contribute valuably to the healthcare team caring for the elderly by assuring that drug therapy is appropriate

References Ø Ø Ø Ø Ø 67 American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2012; DOI: 10. 1111/j. 1532 -55415. 2012. 03923. x Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med 1991; 151: 1825 -32. Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. An Update. Arch Intern Med 1997; 157: 1531 -6. Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med 2003; 163: 2716 -24. State Operations Manual: Appendix PP. Guidance to Surveyors for Long Term Care Facilities, F 329 Unnecessary Drugs, Table 1. rev 1 -7 -2011. Humana, Aetna, Envision, Windsor/CRK Pharmacy Quality Alliance. Use of High-Risk Medications in the Elderly (HRM), Quality Measure, 2013. Proposed 2014 CMS Quality Measures. available at: http: //www. cms. gov/Medicare/Quality-Initiatives. Patient-Assessment-Instruments/Quality. Measures/Downloads/Eligible-Providers-2014 -Proposed. EHR-Incentive-Program-CQM. pdf Clinical Pharmacology, Elsevier/Gold Standard, Copyright 2013, available at: http: //clinicalpharmacology-ip. com accessed 5 -28 -2013. Tapering Recommendations for Medications Commonly Used in the Elderly. Clinical Tools, Geriatric Pharmaceutical Care Guidelines®, Omnicare, Inc. , Cincinnati, OH; 2014. Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the Medical Research Council Cognitive Function and Ageing Study. JAGS 2011; 59: 1477 -83.

Potentially Inappropriate High Risk Medications June 2014

Learning Objectives Ø At the completion of this activity, the 1. identify regulatory and legislative requirements participant will be able to: affecting high risk medications; 2. state mechanisms by which high risk medications are potentially inappropriate in persons older than 65 years of age; and 3. select appropriate monitoring actions and clinical alternatives to high risk medications. 69

Regulatory and Legislative Intersection Centers for Medicare and Medicaid Services Medicare Prescription Drug, Improvement and Modernization Act of 2003 State Operations Manual Appendix PP Skilled Nursing Facility Part D Drug Plan POTENTIALLY UNNECESSARY DRUGS STARS RATING 3

Appendix PP Table 2 – Medications with Significant Anticholinergic Properties Ø Antiparkinson Medications § § § Ø amantadine benztropine trihexphenidyl Muscle Relaxants § § § Ø Ø Ø cyclobenzaprine dantrolene orphenadrine Antivertigo Medications § § meclizine scopolamine Ø § § § Antipsychotic Medications chlorpromazine clozapine olanzapine thioridazine Urinary Incontinence oxybutynin propantheline solifenacin tolterodine trospium Phenothiazine Antiemetics prochlorperazine promethazine Possible side effects from anticholinergic medications include: confusion or decreased cognition, lightheadedness, blurred vision, constipation, dry mouth, and difficulty urinating. CFR 483. 25(I), Unnecessary Drugs 71

Appendix PP Table 2 – Medications with Significant Anticholinergic Properties Antihistamine (H-1 Blockers) Ø Ø § § § § chlorpheniramine cyproheptadine diphenhydramine hydroxyzine Antidepressants amitriptyline amoxapine clomipramine desipramine doxepin imipramine nortriptyline protriptyline paroxetine § § Ø § § § § Cardiovascular Medications digoxin disopyramide furosemide nifedipine Gastrointestinal Medications belladonna chlordiazepoxide cimetidine dicyclomine diphenoxylate with atropine hyoscyamine propantheline ranitidine Possible side effects from anticholinergic medications include confusion or decreased cognition, lightheadedness, blurred vision, constipation, dry mouth, and difficulty urinating. CFR 483. 25(I), Unnecessary Drugs 72

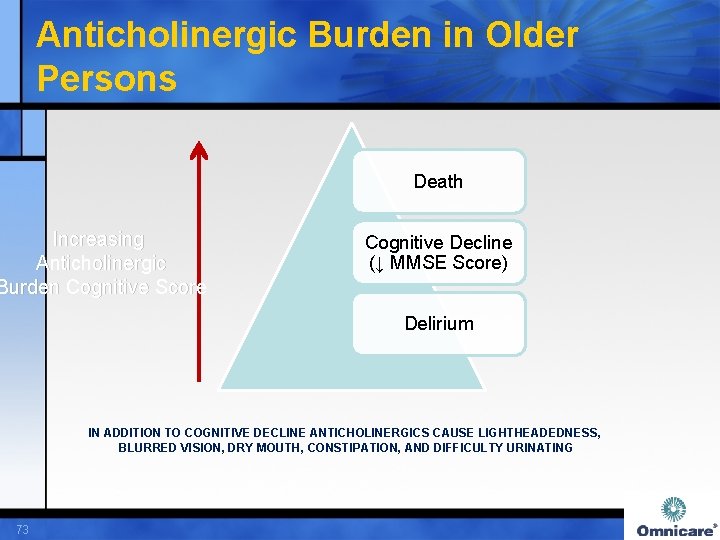
Anticholinergic Burden in Older Persons Death Increasing Anticholinergic Burden Cognitive Score Cognitive Decline (↓ MMSE Score) Delirium IN ADDITION TO COGNITIVE DECLINE ANTICHOLINERGICS CAUSE LIGHTHEADEDNESS, BLURRED VISION, DRY MOUTH, CONSTIPATION, AND DIFFICULTY URINATING 73

Pharmacy Quality Alliance – Use of High Risk Medications in the Elderly PQA • Develops and implements quality performance measures • Measures are adopted by CMS as performance ratings for Medicare Part C and Part D plans 2013 • Use of High Risk Medications in the elderly • % of patients ≥ 65 years who receive 2 or more prescription fills for a high-risk medication during the measurement period. HRM • American Geriatrics Society 2012 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults Pharmacy Quality Alliance, http: // pqaalliance. org/images/uploads/files/HRM%20 Measure%202013 website. pdf Pharmacy Quality Alliance, http: //pqaalliance. org/images/uploads/files/HRM%20 Measure%202013 website. pdf 7

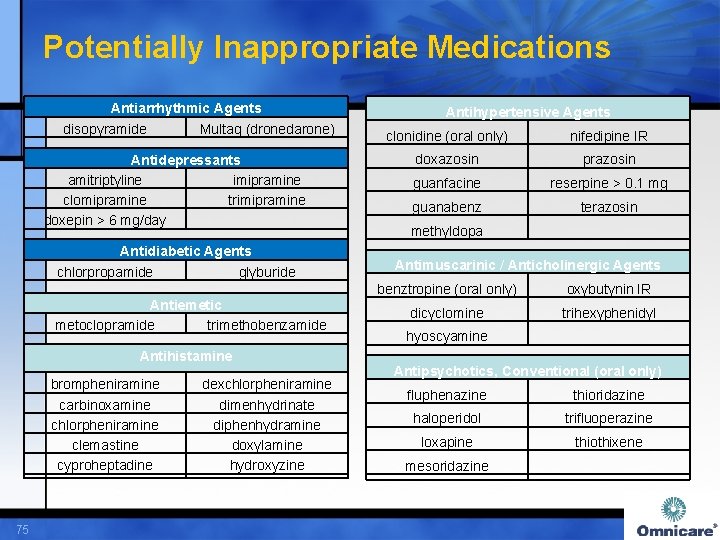
Potentially Inappropriate Medications Antiarrhythmic Agents disopyramide Multaq (dronedarone) Antidepressants amitriptyline imipramine clomipramine trimipramine doxepin > 6 mg/day Antidiabetic Agents chlorpropamide glyburide Antiemetic metoclopramide trimethobenzamide Antihistamine brompheniramine carbinoxamine chlorpheniramine clemastine cyproheptadine 75 dexchlorpheniramine dimenhydrinate diphenhydramine doxylamine hydroxyzine Antihypertensive Agents clonidine (oral only) nifedipine IR doxazosin prazosin guanfacine reserpine > 0. 1 mg guanabenz terazosin methyldopa Antimuscarinic / Anticholinergic Agents benztropine (oral only) oxybutynin IR dicyclomine trihexyphenidyl hyoscyamine Antipsychotics, Conventional (oral only) fluphenazine thioridazine haloperidol trifluoperazine loxapine thiothixene mesoridazine

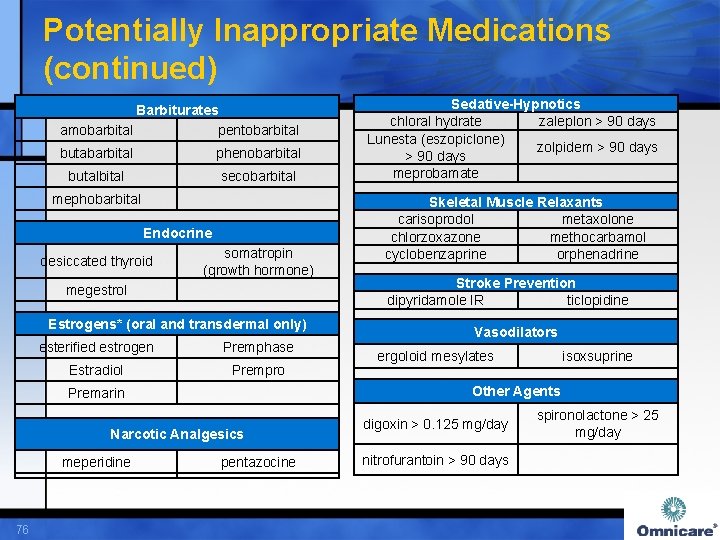
Potentially Inappropriate Medications (continued) Barbiturates amobarbital pentobarbital butabarbital phenobarbital butalbital secobarbital mephobarbital Endocrine desiccated thyroid megestrol somatropin (growth hormone) Estrogens* (oral and transdermal only) esterified estrogen Premphase Estradiol Prempro Narcotic Analgesics 76 Skeletal Muscle Relaxants carisoprodol metaxolone chlorzoxazone methocarbamol cyclobenzaprine orphenadrine Stroke Prevention dipyridamole IR ticlopidine Vasodilators ergoloid mesylates isoxsuprine Other Agents Premarin meperidine Sedative-Hypnotics chloral hydrate zaleplon > 90 days Lunesta (eszopiclone) zolpidem > 90 days meprobamate pentazocine digoxin > 0. 125 mg/day nitrofurantoin > 90 days spironolactone > 25 mg/day

Mechanism, Suggested Monitoring and Potential Alternatives SELECTED HIGH RISK MEDICATIONS 77

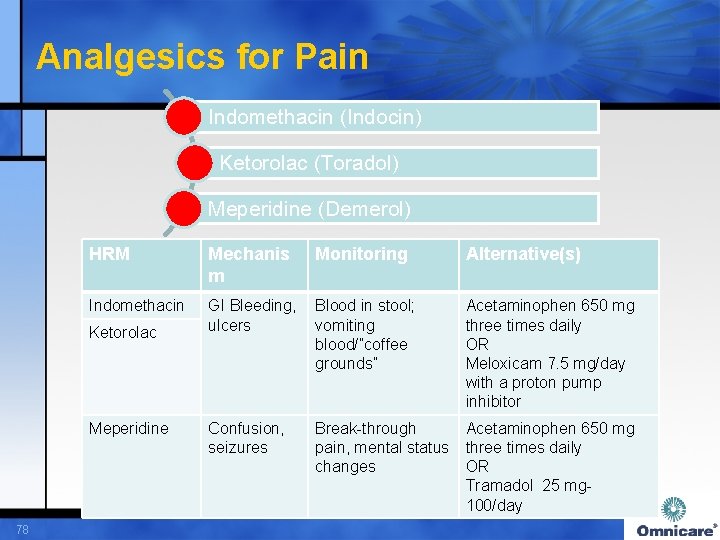
Analgesics for Pain Indomethacin (Indocin) Ketorolac (Toradol) Meperidine (Demerol) HRM Mechanis m Indomethacin GI Bleeding, Blood in stool; ulcers vomiting blood/”coffee grounds” Ketorolac Meperidine 78 Confusion, seizures Monitoring Alternative(s) Acetaminophen 650 mg three times daily OR Meloxicam 7. 5 mg/day with a proton pump inhibitor Break-through Acetaminophen 650 mg pain, mental status three times daily changes OR Tramadol 25 mg- 100/day

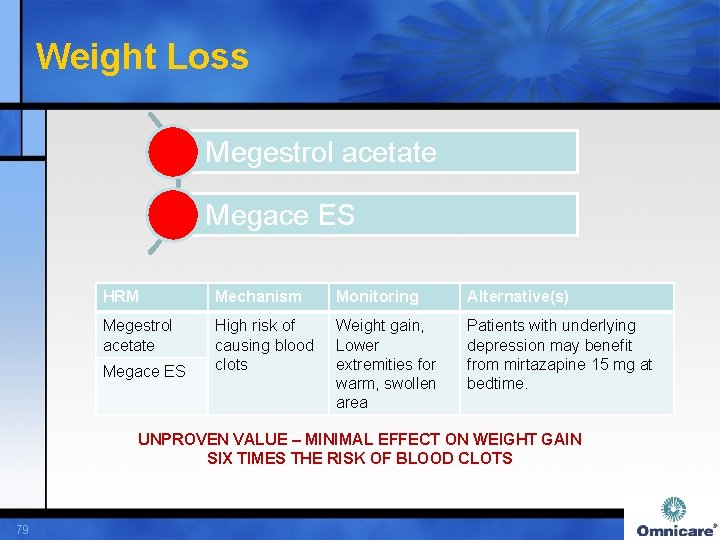
Weight Loss Megestrol acetate Megace ES HRM Mechanism Monitoring Alternative(s) Megestrol acetate High risk of causing blood clots Weight gain, Lower extremities for warm, swollen area Patients with underlying depression may benefit from mirtazapine 15 mg at bedtime. Megace ES UNPROVEN VALUE – MINIMAL EFFECT ON WEIGHT GAIN SIX TIMES THE RISK OF BLOOD CLOTS 79

First Generation Antihistamines Brompheniramine, Dexchlorpheniramine Carbinoxamine, Chlorpheniramine Clemastine, Cyproheptadine Diphenhydramine Doxylamine Hydroxyzine, Hydroxyzine pamoate Triprolidine HRM Mechanism Sedating Anticholinergi Antihistamine c s 80 Monitoring Alternatives Confusion, dry mouth, constipation, dizziness, falls Loratadine 10 mg/day for seasonal allergy and itching. Buspirone 5 mg twice daily for anxiety

Cardiovascular Agents – Blood Pressure Clonidine Nifedipine IR Methyldopa Reserpine > 0. 1 mg/day HRM Mechanism Monitoring Alternative(s) Clonidine CNS side effects, bradycardia, hypotension, myocardial ischemia Lying to sitting and sitting to standing BP, mental status changes, heart rate Lisinopril 2. 5 mg/day Losartan 25 mg/day Nifedipine IR Methyldopa Reserpine 81 CNS side effects, bradycardia NOT FIRST-LINE FOR HYPERTENSION

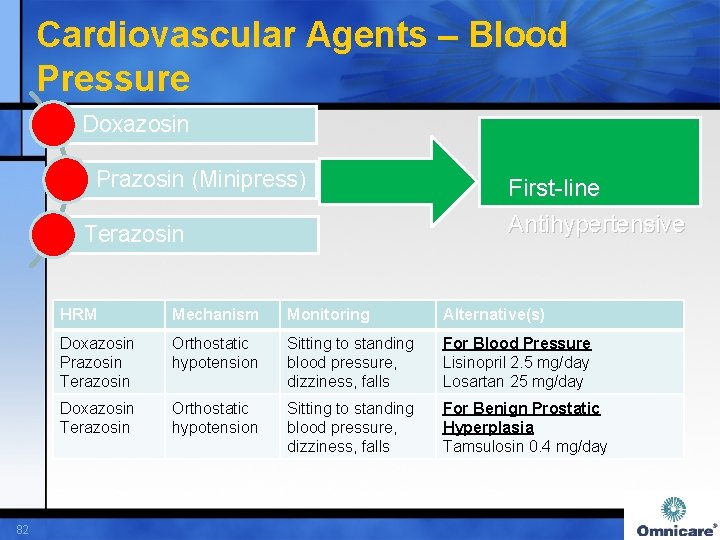
Cardiovascular Agents – Blood Pressure Doxazosin Prazosin (Minipress) Terazosin 82 First-line Antihypertensive HRM Mechanism Monitoring Alternative(s) Doxazosin Prazosin Terazosin Orthostatic hypotension Sitting to standing blood pressure, dizziness, falls For Blood Pressure Lisinopril 2. 5 mg/day Losartan 25 mg/day Doxazosin Terazosin Orthostatic hypotension Sitting to standing blood pressure, dizziness, falls For Benign Prostatic Hyperplasia Tamsulosin 0. 4 mg/day

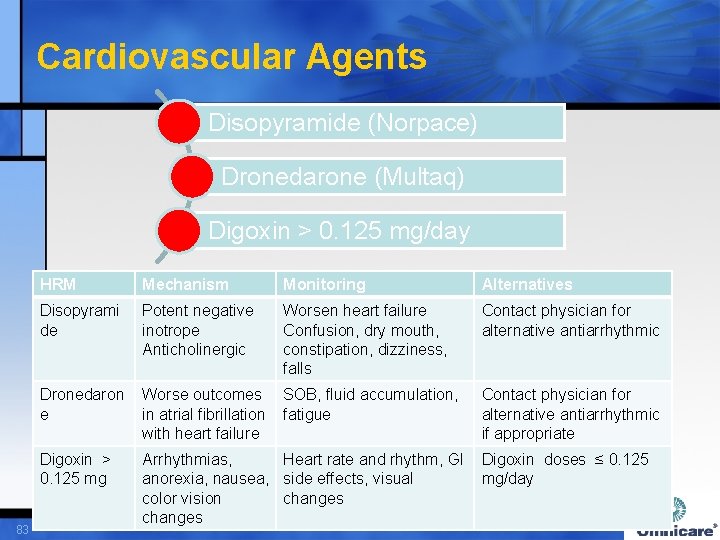
Cardiovascular Agents Disopyramide (Norpace) Dronedarone (Multaq) Digoxin > 0. 125 mg/day 83 HRM Mechanism Monitoring Alternatives Disopyrami de Potent negative inotrope Anticholinergic Worsen heart failure Confusion, dry mouth, constipation, dizziness, falls Contact physician for alternative antiarrhythmic Dronedaron e Worse outcomes SOB, fluid accumulation, in atrial fibrillation fatigue with heart failure Digoxin > 0. 125 mg Arrhythmias, Heart rate and rhythm, GI Digoxin doses ≤ 0. 125 anorexia, nausea, side effects, visual mg/day color vision changes Contact physician for alternative antiarrhythmic if appropriate

Anti-nauseant/Anti-emetic Medications Promethazine and combination products Trimethobenzamide $$$ $ HRM Mechanism Promethazine Anticholinergic Confusion, dry Ondansetron 4 mg twice mouth, constipation, daily up to a Max Daily dizziness, falls Dose – 24 mg/day Trimethobenzamid Extrapyramida e l effects 84 Monitoring AIMS Testing Alternative(s)

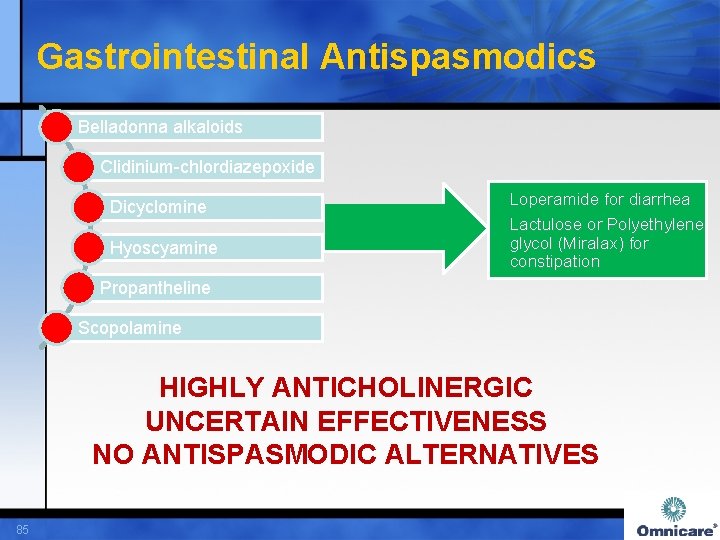
Gastrointestinal Antispasmodics Belladonna alkaloids Clidinium-chlordiazepoxide Dicyclomine Hyoscyamine Loperamide for diarrhea Lactulose or Polyethylene glycol (Miralax) for constipation Propantheline Scopolamine HIGHLY ANTICHOLINERGIC UNCERTAIN EFFECTIVENESS NO ANTISPASMODIC ALTERNATIVES 85

Diabetes Mellitus • Chlorpropamide Long-acting • Glyburide Sulfonylurea Mechanism • Prolonged hypoglycemia • Inappropriate antidiuretic hormone secretion • Glipizide 5 mg/day up to 20 mg/day • Glimepiride 1 mg/day up to 8 mg/day Alternatives • Basal insulin with scheduled prandial insulin as needed AVOID THE USE OF SLIDING SCALE INSULIN 86

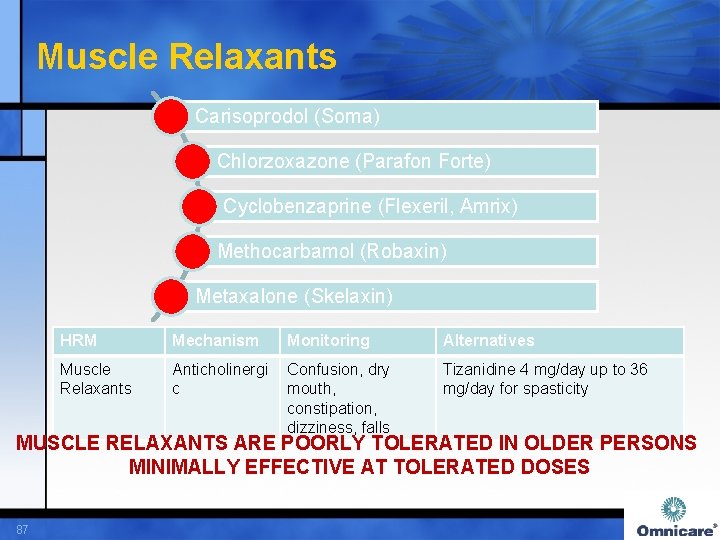
Muscle Relaxants Carisoprodol (Soma) Chlorzoxazone (Parafon Forte) Cyclobenzaprine (Flexeril, Amrix) Methocarbamol (Robaxin) Metaxalone (Skelaxin) HRM Mechanism Monitoring Alternatives Muscle Relaxants Anticholinergi c Confusion, dry mouth, constipation, dizziness, falls Tizanidine 4 mg/day up to 36 mg/day for spasticity MUSCLE RELAXANTS ARE POORLY TOLERATED IN OLDER PERSONS MINIMALLY EFFECTIVE AT TOLERATED DOSES 87

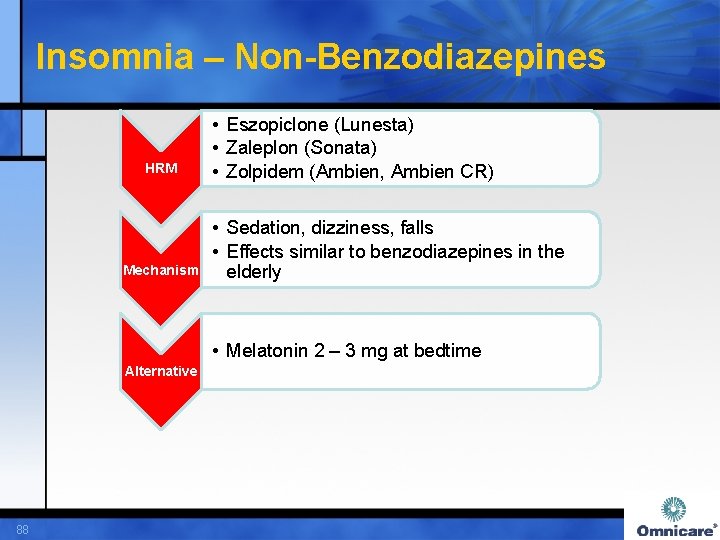
Insomnia – Non-Benzodiazepines HRM Mechanism • Eszopiclone (Lunesta) • Zaleplon (Sonata) • Zolpidem (Ambien, Ambien CR) • Sedation, dizziness, falls • Effects similar to benzodiazepines in the elderly • Melatonin 2 – 3 mg at bedtime Alternative 88

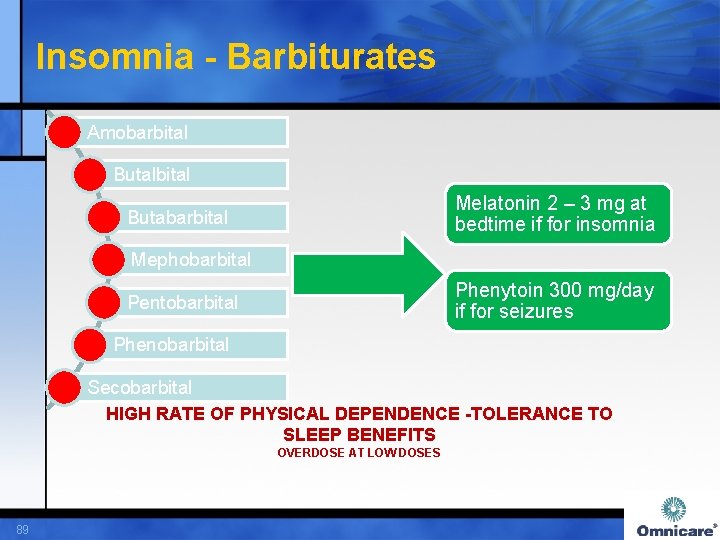
Insomnia - Barbiturates Amobarbital Butalbital Melatonin 2 – 3 mg at bedtime if for insomnia Butabarbital Mephobarbital Phenytoin 300 mg/day if for seizures Pentobarbital Phenobarbital Secobarbital HIGH RATE OF PHYSICAL DEPENDENCE -TOLERANCE TO SLEEP BENEFITS OVERDOSE AT LOW DOSES 89

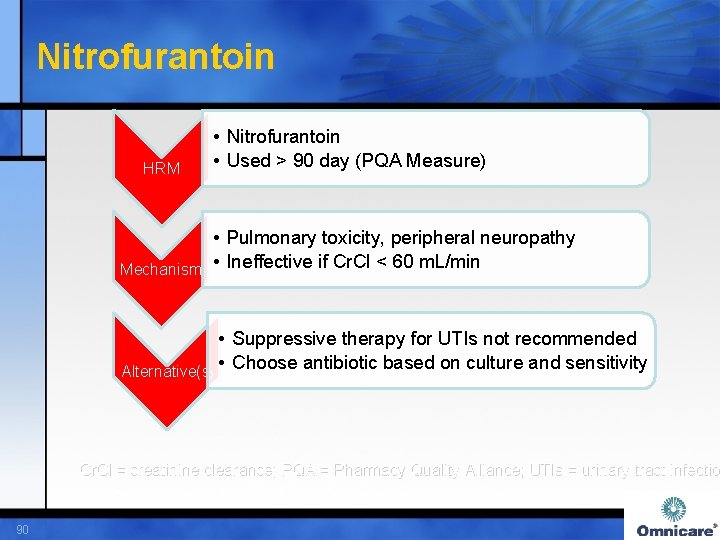
Nitrofurantoin HRM • Nitrofurantoin • Used > 90 day (PQA Measure) • Pulmonary toxicity, peripheral neuropathy Mechanism • Ineffective if Cr. Cl < 60 m. L/min • Suppressive therapy for UTIs not recommended Alternative(s) • Choose antibiotic based on culture and sensitivity Cr. Cl = creatinine clearance; PQA = Pharmacy Quality Alliance; UTIs = urinary tract infectio 90

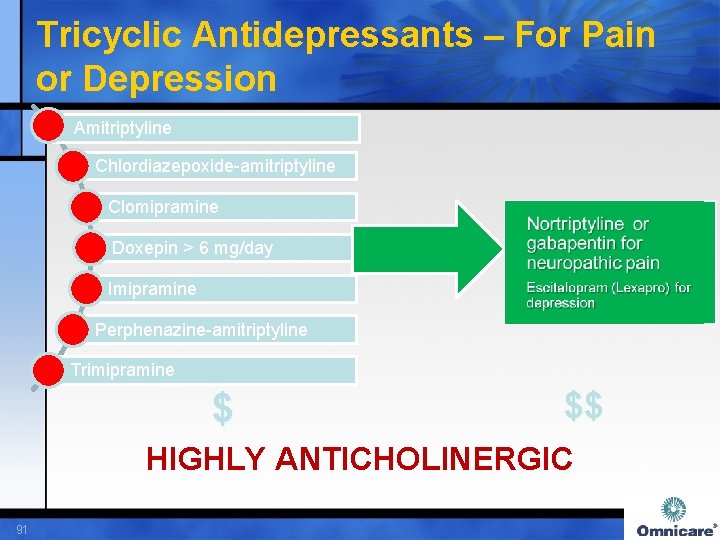
Tricyclic Antidepressants – For Pain or Depression Amitriptyline Chlordiazepoxide-amitriptyline Clomipramine Doxepin > 6 mg/day Imipramine Perphenazine-amitriptyline Trimipramine $ $$ HIGHLY ANTICHOLINERGIC 91

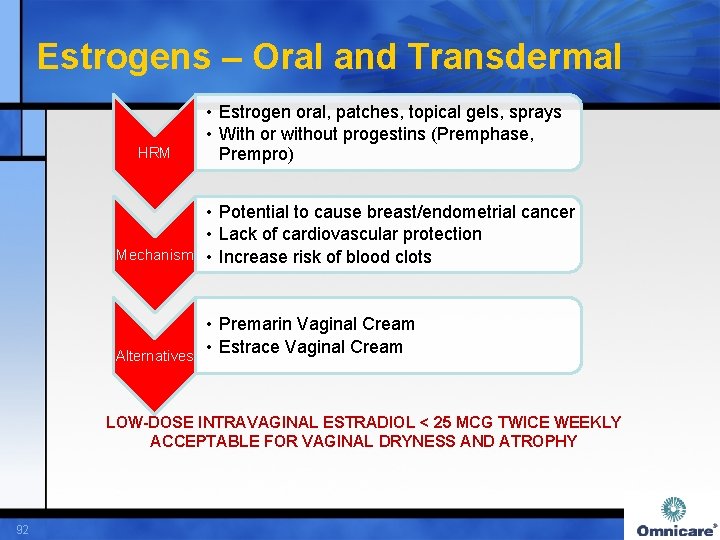
Estrogens – Oral and Transdermal HRM • Estrogen oral, patches, topical gels, sprays • With or without progestins (Premphase, Prempro) • Potential to cause breast/endometrial cancer • Lack of cardiovascular protection Mechanism • Increase risk of blood clots • Premarin Vaginal Cream • Estrace Vaginal Cream Alternatives LOW-DOSE INTRAVAGINAL ESTRADIOL < 25 MCG TWICE WEEKLY ACCEPTABLE FOR VAGINAL DRYNESS AND ATROPHY 92

General Guidance on Discontinuation Many medications can be discontinued abruptly without adverse consequences. e. g. estrogens, antihistamines, oxybutynin, megestrol Most medications with central nervous system properties must be tapered then discontinued. e. g. tricyclic antidepressants, insomnia meds, barbiturates, muscle relaxants, anticonvulsants, clonidine, tramadol Some medications should be tapered to reduce the risk of worsening a patient’s condition. e. g. antipsychotics, antidepressants TAPERING GUIDANCE IS ON OMNIVIEW IN THE GERIATRIC PHARMACEUTICAL CARE GUIDELINES 93

Summary High Risk Medications High risk of clinical adverse consequences for residents , i. e. , falls, declines in ADLs Regulated by CMS - Declining Payment by Part D Plans Increased risk of survey citation for F-329 Unnecessary Drugs Increased facility cost for non-covered high risk medications Safer and More Appropriate Alternatives Exist Some alternatives may be priced higher than high risk medication 94 Reduction in risk of toxicity and adverse events results in fewer hospitalizations